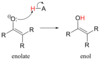Chapter 5 - Carbonyls and Alcohols Flashcards
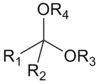
Acetal structure

Hemiacetal structure

Ketal structure
Hemiketal structure

Acid halide structure

Acid anhydride structure
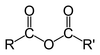
Amide structure

Imide structure

Lactone structure

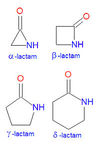
Lactam structure
Describe the signal present for a hydroxyl group on 1H NMR
broad peak between 1 and 5 ppm
Describe the general reactivity of alcohols
They are not good nucleophiles, but they can be deprotonated and converted into an alkoxide under basic conditions.
Describe the general reactivity of aldehydes and ketones
They are reactive with most nucleophiles , but not by a traditional nucleophilic substitution mechanism. The reactivity occurs mainly at the electrophilic carbonyl center, often forming a tetrahedral intermediate.
Out of ketals, hemiketals, acetals, and hemiacetals, which can be formed and removed under acidic conditions?
acetals and ketals
Out of ketals, hemiketals, acetals, and hemiacetals, which can be formed only under basic conditions, but removed under any conditions?
hemiacetals and hemiketals
What are the 4 steps in an acid-catalyzed mechanism?
1) protonate (making the leaving group a better leaving group)
2) break bond (leaving group leaves)
3) make bond (nucleophile attacks carbocation)
4) deprotonate (molecule returns to natural state)
What are the 4 steps in a base-catalyzed mechanism?
1) deprotonate (make a strong nucleophile)
2) make bond (nucleophile attacks), break bond (leaving group leaves)
3) protonate (molecule returns to neutral state)
What charge do intermediates carry in an acid-catalyzed mechanism?
positive
What charge do intermediates carry in a base-catalyzed reaction?
negative
The addition of an alcohol to an aldehyde under acidic conditions yields what?
an acetal
The addition of an alcohol to a ketone under basic conditions yields what?
a hemiketal
What reactant is needed to form a ketal or acetal protecting group?
a 1,2-diol (OHCH2CH2OH) under acidic conditions

What is produced when an ester is reacted with a strong base in water?
Saponification: forms a carboxylate (deprotonated carboxylic acid) + alcohol

What is produced when an ester is reacted with an acid in water?
Ester hydrolysis: carboxylic acid + alcohol
What is formed when a methyl ketone is reacted with I2 and strong base and water?
Iodoform reaction: carboxylate (deprotonated carboxylic acid) + I3CH (iodoform)
What is formed when an aldehyde is reacted with potassium permanganate (KMnO4) in strong base and water?
Oxidation: carboxylic acid
What is formed when a primary alcohol is reacted with potassium dichromate (K2Cr2O7) and acid in water?
Oxidation: carboxylic acid
What is formed when a secondary amide is reacted with a strong acid at high temperature?
Amide hydrolysis: carboxylic acid + RNH3+
What is formed when a nitrile is reacted with a strong acid at high temperature?
Nitrile hydrolysis: carboxylic acid + NH4+
What is formed when a carboxylic acid is reacted with thionyl chloride (SOCl2)?
acid halide
What is formed when a carboxylic acid is reacted with an alcohol and acid in high temperature?
transesterification: ester
What is formed when a carboxylic acid is treated with high temperature?
Condensation reaction: acid anhydride
What is formed when a carboxylic acid is reacted with 1) LiAlH4 (lithium aluminum hydride), 2) NH4Cl (ammonium chloride)?
primary alcohol
Why are esters more reactive than ketones/aldehydes?
They have an alkoxy (OR) leaving group.
What is formed when an ester is reacted with alcohol at high temperature under acidic conditions?
Transesterification: new ester with an alkoxy group from the alcohol
What is formed when a cyclic ketone is reacted with peroxyacids (RCO3H) (commonly mcpba)?
Baeyer-Villiger reaction: lactone
What is formed when an acid anhydride is reacted with water?
Hydrolysis: 2 carboxylic acids
Why are acid halides the most reactive of all the carbonyl groups?
They have the best leaving group.
What functional group makes up the peptide bond?
amide
What is formed when an amide is reacted with LiALH4 (lithium aluminum hydride)?
Reduction: amine
What functional group forms the hydrogen bond in base pairing of DNA and RNA?
amide
Carbonyl reactions can be categorized into what 3 categories?
- attack at carbonyl carbon
- deprotonation of alpha proton
- oxidation/reduction
What are the 2 categories of reactions in which a nucleophile attackes the electrophilic carbonyl carbon?
- substitution (leaving group)
- addition (no leaving group)
What determines a carbonyl’s leaving group strength?
the pKa of its conjugate acid (good leaving groups have better stronger conjugate acids with low pKas)
decreasing order of reactivity for acid anhydride, acid halide, amide, ester
acid halide > acid anhydride > ester > amide
What forms in the deprotonation of alpha protons?
enolates