Chapter 20: The Kidney - Tubular/interstitial Dz/Vascular Dz Flashcards
Which stain is used to view Chronic Glomerulonephritis?
What’s seen?
- Massone trichrome stain
- Showing complete replacement of all glomeruli by blue-staining collagen

90% of what type of glomerulonephritis will progress to chronic GN?
Other forms of GN that may progress?
- Crescentic GN (90%)
- Membranous nephropathy
- FSGS
- Membranoproliferative GN

As glomeruli progressively become obliterated in chronic GN, what happens to GFR and protein loss?
- GFR decreases
- Protein loss in urine diminshes
What is the characteristic morphological features of the glomerular lesions associated with SLE (lupus nephritis)?
What is seen on light microscopy and electron microscopy?
- SUBendothelial immune complex deposition + mesangial deposition
- GBM shows “wire loops” of capillaries on light microscopy

What are the 2 major processes that play a key pathophysiologic role as diabetic glomerular lesions develop?
- Metabolic defect linked to hyperglycemia and advanced glycosylation end products produced thickened GBM and increased mesangial matrix
- Hemodynamic effects associated w/ glomerular hypertrophy also contribute to the development of glomerulosclerosis
What are the 3 major morphological changes seen in diabetic glomerulosclerosis?
- Diffuse capillary BM thickening
- Diffuse mesangial sclerosis
- Nodular glomerulosclerosis****

In the US, what is the leading cause of end-stage renal disease, adult-onset blindness and non-traumatic LE amputations?
Diabetes
What are the major characteristics of Henoch-Schonlein Purpura?
Who does it most typically affect and onset when?
- Most common in children btw 3-8 yo, often follows a URI
- Purpuric skin rashes on extensor surfaces of arms and legs
- Abdominal pain and vomiting —> intestinal bleeding
- Arthralgias
- Renal abnormalities - microscopic hematuria, mixed or isolated nephritic/nephrotic syndrome
Pathognomonic feature of Henoch-Schonlein Purpura seen on fluorescence microscopy?
Deposition of IgA, sometimes w/ IgG and C3, in mesangial region
What is the morphology of the skin lesions seen in Henoch-Schonlein Purpura?
- Subepidermal hemorrhages and necrotizing vasculitis involving smaller vessels of the dermis
- Deposition of IgA, along w/ IgG and C3 present in the vessels
Which 4 systemic diseases are associated w/ Nephrotic Syndrome?
- Diabetic nephropathy
- SLE
- Hepatitis C - Cryoglobulinemia: Membranoproliferative Type I
- HIV nephropathy: FSGS
Which 4 systemic diseases are associated w/ Nephritic Syndrome?
- SLE = 60-70% pts
- Bacterial endocarditis: acute proliferative GN
- Goodpasture Syndrome: RPGN
- Henoch-Schonlein Purpure (HSP): IgA nephropathy
What are the 3 major renal lesions prototypical of Diabetic Nephropathy?
- Glomerular lesions
- Vascular lesions, principally artriolosclerosis
- Pyelonephritis, including necrotizing papillitis
In diffuse proliferative lupus nephritis what will be seen with immunofluorescence?
Mesangial and capillary wall (SUBendothelial) IgG localization

Out of the 6 patterns of glomerular disease seen in SLE, which is the most common?
Common signs/sx’s in these pts?
- Diffuse lupus nephritis (class IV)
- Hematuria and proteinuria.
- HTN and mild to severe renal insufficiency is also common
What is the most common cause of acute kidney injury (aka ARF)?
Acute tubular injury/Necrosis
What are the 2 most common etiologies causing Acute Tubular Injury?
- Ischemia i.e., hypotension, shock, HUS, TTP, or DIC
- Direct toxic injury to the tubules (drugs/toxins)

How does the pattern of tubular damage in the PCT, PST, and ascending loop of henle seen in acute tubular injury differ between ischemic and toxic sources?
- Ischemic = patchy necrosis of these segments
- Toxic = continous necrosis of PCT and PST w/ patchy necrosis of ascending loop of henle

Why are tubular epithelial cells particularly vulnerable to ischemia and toxins?
- Increased SA for reabsorption
- High rate of metabolism + O2 consumption w/ high energy requirements –> ischemia disrupts this
In ATI, what occurs to necrotic tubular epithelial cells over time?
Leads to?
Detach and sloughed into tubular lumen –> luminal obstruction by casts

What is the histologic findings of acute kindey injury associated w/ ethylene glycol (aka anti-freeze)?
Often find what type of crystals in the tubular lumen?
- Marked ballooing and hydropic or vacuolar degeneration of PCT’s
- Calcium oxalate crystals

What are the 3 stages of the clinical course of AKI/ARF and major electrolyte/lab findings in each stage?
Which stage is marked by an increases susceptibility to infection?
- Initiation - lasts about 36 hrs w/ slight decline in urine output + rise in BUN
- Maintenance - salt + H2O overload, rising BUN, and HYPERkalemia, metabolic acidosis, OLIGURIA
- Recovery - HYPOkalemiabecomes an issue as well asincreased susceptibility to infection

What is the prognosis of ATI dependent on?
How likely are these pts to survive?
- Depends on magnitude and duration of injury
- With current supportive care, 95% of those who do not succumb to the precipitating cause recover!
What are the clinical hallmarks of Tubulointerstitial Nephritis that distinguish it from Glomerular diseases?
- Absence of nephritic or nephrotic syndrome
- Defects in tubular function –> defect in concentrating urine = polyuria and nocturia
- Salt wasting
- Dimished ability to excrete acids (metabolic acidosis)
Tubulointerstitial Nephritis is generally characterized by presence of what functional renal abnormality?
- Azotemia –> ↓ GFR
- Inability to concentrate urine –> polyuria and nocturia
Most common cause of clinical pyelonephritis arises from what?
Ascending infection from the bladder
Pyelonephritis is defined as inflammation affecting what anatomical features of the kidney?
- Tubules
- Interstitium
- Renal pelvis
What is the second most common cause of acute kidney injury (after pyelonephritis)?
Drug and toxin-induced tubulointerstitial nephritis
A small % of pts w/ analgesic nephropathy develop which malignancy?
Urothelial carcinoma of the renal pelvis
What are the major clinical signs/sx’s of acute drug-induced interstitial nephritis?
Onset?
- Begins about 15 days after drug exposure
- With fever, eosinophilia (transient), a rash, and renal abnormalities (i.e., hematuria, mild proteinuria, and leukocyturia)
What is one of the early and reversible results of ischemia associated w/ tubule cell injury?
- Loss of cell polarity
- Redistribution of Na-K-ATPase from basolateral to luminal surface of tubular cells
- Increase Na+ delivery to distal tubules
What is the major hemodynamic alteration leading to a reduction in GFR associated with ischemic tubule cell injury?
- Intrarenal vasoconstriction
- Results in reduced glomerular flow and ↓O2 delivery to functionally important tubules (TAL and PT)
ATI is characterized by what type of cell death and morphological findings?
- Focal tubular epithelial necrosis at multiple points of nephron
- Occlusion of tubular lumens by casts

What are the eosiniphilic hyaline and pigmented granular casts seen with ATI composed of?
Tamm-Horsfall protein
What findings when present can help distinguish acute from chronic interstitial fibrosis?
- In acute, there will be more rapid clinical onset w/ edema
- Often w/ presence of neutrophils and eosinophils
Which 3 viruses may be responsible for UTI’s/renal infection, especially in immunocompromised pts such as those w/ transplants?
1) Polyomavirus
2) CMV
3) Adenovirus
What are 2 of the common predisposing medical conditions for Pyelonephritis?
1) Diabetes –> especially poorly controlled
2) Pregnancy
Which anatomic defect is an important predisposing factor for ascending infection leading to pyelonephritis?
- Incompetence of the vesicouretal valve
- Leads to reflux of urine from bladder –> ureter = vesicoureteral reflex
How can vesicoureteal reflux be acquired in both children and adults?
- Children –> congenital defect (most common) or acquired by bladder infection
- Adults –> may be acquired from persistent bladder atony due to spinal cord inury
How can vesicoureteral reflux by diagnosed?
Voiding cystourethrogram

Although ascending infection is the most common route of bacteria entering the kidney, they may also do so hematogenously, which is most often occurs in what clinical setting?
Ongoing sepsis

What are the morphological hallmarks of Acute Pyelonephritis?
- Patchy interstitial suppurative inflammation
- Intratubular aggregates of neutrophils
- Neutrophilic tubulitis and tubular necrosis

Acute pyelonephritis usually shows sparing of the glomeruli, but infection by what often destroys glomeruli?
Fungal pyelonephritis (i.e., Candida) –> granulomatous interstitial inflammation
Papillary necrosis is a complication of acute pyelonephritis most often seen in which 4 patients/settings?
1) Diabetics
2) Analgesic Nephropathy
3) Urinary tract obstruction
4) Sickle Cell Disease

Is papillar necrosis usually unilateral or bilateral and what type of morphological damage is seen?
- Typically bilateral but can be unilateral
- Tips/distal 2/3’s of pyramids have areas of gray-white to yellow necrosis (ischemic coagulative necrosis)

What is the M:F ratio for papillary necrosis associated with diabetes mellitus?
Time course?
How do the papillae appear and are they calcified?
- Females 3:1
- Time course = 10 yrs
- Pale grayish necrosis limited to papillae; calcifications = rare

What is the M:F ratio for papillary necrosis associated with Analgesic Nephropathy?
Time course of abuse?
How does the necrosis appear on the papillae, how diffuse is the necrosis and is there calcification?
- Females 5:1
- Time course = 7 years of abuse
- Almost ALL papillae in DIFFERENT stages red-brown necrosis w/ sloughing into calyces; calcifications are frequent

Which gender has a higher incidence of papillary necrosis as a result of obstruction?
Are calcifications rare or frequent?
- Males 9:1
- Calcifications = frequent

After the acute phase of pyelonephritis, healing occurs, with scarring commonly seen in which type of pattern?
Pyelonephritic scars are almost always associated w/ inflammation, fibrosis, and deformation of which structures?
- Patchy, jigsaw pattern w/ intervening preserved parenchyma
- Scar associated w/ underlying calyx and pelvis
What is the common clinical presentation of acute pyelonephritis (signs/sx’s)?
- Sudden onset of pain at costovertebral angle + fever and malaise
- Often dysuria, frequency, and urgency indicating bladder/urethral irritation
Which finding in the urine is indicative of renal involvement in a patient presenting w/ signs of acute pyelonephritis?
Leukocyte casts, typically rich in neutrophils (pus casts)
What is an emerging viral pathogen causing pyelonephritis in kidney allografts often leading to the development of nephropathy/allograft failure?
Polyomavirus (i.e., JC or BK virus)
Only what 2 entities causing renal damage affect the calyces, making pelvocalyceal damage an important diagnostic clue?
1) Chronic pyelonephritis
2) Analgesic nephropathy
What are the 2 major forms of Chronic Pyelonephritis?
Which is most common?
- Reflux nephropathy = MOST common
- Chronic obstructive pyelonephritis
What is sterile reflux and what is it caused by?
- Renal damage by vesicoureteral reflux in absence of infection
- Due to severe obstruction
Xanthogranulomatous pyelonephritis is a rare form of chronic pyelonephritis characterized by the accumulation of what?
Associated with what infection?
The large, yellowish orange nodules may be clinically confused with?
- Foamy macrophages w/ plasma cells, lymphocytes, PMN’s, and occasional giant cells
- Often w/ Proteus infections
- Large, yellowish orange nodules may be confused w/ renal cell carcinoma
How does the scarring/symmetry of chronic pyelonephritis differ from that of chronic glomerulonephritis?
- Chronic pyelonephritis = irregular scarring w/ asymmetry if bilateral
- Chronic GN = diffuse and symmetrical scarring
Coarse, discrete, corticomedullary scars overlying dilated, blunted, or deformed calyces w/ flattening of papillae most often in upper and lower poles is the hallmark of what disorder?
Chronic pyelonephritis
Dilated tubules w/ flattened epithelium often filled w/ casts resembling thyroid colloid (thyroidization) is characteristic of what disorder of the kidney?
Chronic pyelonephritis
Reflux nephropathy as a contributor to chronic pyelonephritis is often discovered in kids when?
Late in the disease as renal insufficiency and HTN develop, often as the cause of HTN in a child is being investigated
What secondary disorder may develop due to increased scarring in pts w/ chronic pyelonephritis?
Presence of what is a poor prognostic sign in these pts?
- FSGS
- Onset of proteinuria + FSGS = poor prognostic sign, which may progress to ESRD
The most likely sequence of events in acute drug-induced interstitial nephritis is that drugs act as what?
Leads to?
- Drugs act as haptens binding to components of tubular cells
- Become immunogenic w/ resulting injury due to IgE or cell-mediated immune rxns to tubular cells or their BM’s
Why is it clinically important to recognize drug-induced acute interstitial nephritis?
Withdrawl of the offending drug is followd by recovery, which may take several months
On occasaion, necrotic papillae are excreted in the urine and may cause what clinical signs/sx’s?
- Gross hematuria
- Renal colic due to ureteric obstruction
NSAIDs have been shown to cause what 2 renal syndromes developing concurrently?
- Acute interstitial nephritis
- Minimal change disease
Acute uric acid nephropathy often seen in which patients, receiving what therapy?
- Those w/ leukemias or lymphomas undergoing chemotherapy (tumor lysis syndrome)
- Released nuclei acids –> uric acid (precipitation favored by acidic pH in collecting tubules)
Nephrolithiasis vs. nephrocalcinosis?
Disorders associated w/ each?
- Nephrolithiasis = uric acid stones seen in pts w/ gout and secondary hyperuricemia
- Nephrocalcinosis = deposits of calcium in the kidney (NOT stones) associated w/ hyperparathyroidism, multiple myeloma, metastatic cancer, and vit D intoxication
What is the main cause of renal dysfunction in pts w/ Multiple Myeloma?
Bence-Jones (light-chain) proteinuria
What are the 2 mechanism which account for the renal toxicitiy of Bence-Jones (light-chain) proteins in Multiple Myeloma pts?
1) Some Ig light chains are directly toxic to epithelial cells
2) Combine w/ urinary glycoproteins (Tamm-Horsfall) under acidic conditions to form large tubular casts, obstructing tubular lumens inducing inflammation = light-chain cast nephropathy
What are the 4 main ways that renal damage can occur in Multiple Myeloma pts?
- Bence-Jones proteinuria and cast nephropathy
- Amyloidosis
- Light-chain deposition disease
- Hypercalcemia and Hyperuricemia
What is light-chain deposition disease seen in some pts with multiple myeloma?
- Light chains (usually K type) deposit in GBMs and mesangium causing a glomerulopathy
- Also in tubular BM’s which may cause tubulointerstitial nephritis
What is the characteristic tubulointerstitial morphology seen in light-chain cast nephropathy?
- Blue amorphous masses, sometimes concentrically laminated and fractured, filling and distending tubular lumens
- Some casts surrounded by multinucleate giant cells

If a patient with multiple myeloma presents w/ significant non-light chain proteinuria (i.e., albuminuria) what type of nephrotic disease process may this suggest?
- AL amyloidosis
- Light-chain deposition disease
Clinically, what is the most common presentation for the renal manifestations associated with light-chain cast nephropathy?
1) CKD developing slow and progressively = most common
2) AKI w/ oliguria = less common
Hepatorenal syndrome seen in pts w/ acute or chronic liver disease w/ advanced liver failure may cause what type of nephropathy?
Bile cast nephropathy
Nephroslcerosis is defined as sclerosis of what vessels in the kidney?
Strongly associated with what disease?
- Renal arterioles and small arteries
- Strong association w/ HTN
*Benign nephrosclerosis is a general process, NOT a specific Dx
Nephrosclerosis is associated with what factors?
- Advancing age
- Blacks > whites
- Strongly associated w/ HTN and DM; may be seen in absence of HTN too
What are the 2 processes occuring in the pathogenesis of Benign Nephrosclerosis?
- Medial and intimal thickening due to hemodynamic changes, aging, etc
- Hyalinization of arteriolar walls –> extravasation of plasma protein thru injured endothelium and increased deposition in BM’s
What is the gross morphology (i.e., cortical surface) of the kidneys in Benign Nephrosclerosis?
- Cortical surface is fine w/ granularity (“resembles leather”) due to cortical scarring and shrinking
- Will be of normal size or moderately reduced in size

Benign nephrosclerosis is NOT usually associated w/ renal insufficiency except in which 3 groups of pts w/ HTN?
- Pts of African descent
- Pts w/ severe BP elevations
- Pts w/ 2nd underlying dz, especially diabetes
What are histological features of the kidneys seen in Benign Nephrosclerosis?
- Hyaline arteriolsclerosis
- Fibroelastic hyperplasia
- Patchy ischemic atrophy of tubules and glomeruli
Malignant Nephrosclerosis typically occurs most often in whom?
- Younger pts
- More often in men and in blacks
What are the 2 histologic alterations that characterize blood vessels in malignant HTN (Nephrosclerosis)?
Which of these changes correlates w/ renal failure?
- Fibrinoid necrosis of arterioles
- Hyperplastic arteriolosclerosis (AKA “Onion skinning”) = proliferation of smooth m. cells of the interlobular arteries –> correlates w/ renal failure

Explain the pathogenesis of the lesions in Malignant Nephrosclerosis after the initial vascular damage.
- Injured endothelium = ↑ permeability to fibrinogen + plasma proteins
- Focal cell death + platelet deposition –> fibrinoid necrosis of arterioles and small arteries
- Activation of platelets + coagulation factors –> intravascular thrombosis
- Hyperplastic arteriolosclerosis –> marked ischemia of kidneys

How does the cortical surface of the kidney appear in Malignant Nephrosclerosis?
Small, pinpoint petechial hemorrhages = “flea-bitten” appearance

Patients w/ malignant HTN have markedly elevated levels of?
Leads to what type of cycle?
- Renin
- Self-perpetuating cycle of damage and HTN
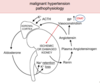
The full-blown syndrome of Malignant HTN is characterized by what BP, and other serious clinical manifestations?
- BP = >200 / >120 mmHg
- Papilledema
- Retinal hemorrhages
- Encephalopathy
- CV abnormalities
- Renal failure
Most often the early symptoms of Malignant HTN include what?
What renal findings?
- Sx’s related to ↑ ICP –> HA, N/V, and visual impairments (i.e., scotomas or seeing spots)
- Marked proteinuria and micro/macroscopic hematuria
What is the importance clinically in recognizing Renal Artery Stenosis in regards to treatment?
- Potentially curable form of HTN = 70-80% cure rate
Renal artery stenosis leads to what pathophysiologic changes?
- Increased prod. of renin from ischemic kidney –> HTN
- Sodium retention may occur and perpetuate the HTN
What is the most common cause of renal artery stenosis (70%)?
Who is most at risk?
- Narrowing at origin of renal artery by atheromatous plaque
- More frequent in men, ↑ with advanced age, and diabetes
Which cause of renal artery stenosis is more often seen in younger age groups (3rd-4th decades) and is more common in woman?
Fibromuscular dysplasia of renal artery

What is the classic appearance on arteriography of renal artery stenosis due to fibromuscular dysplasia?
“String of beads” appearance

In general pts w/ renal artery stenosis present clinically similar to what other disorder?
How can it be diagnosed?
- Resemble those w/ essential HTN
- Occasionally bruit can be heard over kidney on ausculation (rare)
- Elevated renin levels, response to ACE inhibitors, renal scans, and IV pyelography may all aid in Dx
- Need arteriography to localize stenotic lesion
What is the arteriolosclerosis like in the ischemic kidney in renal artery stenosis vs. non-ischemic (functional) kidney?
- Ischemic kidney will be reduced in size and show signs of diffuse ischemic necrosis
- Contralateral kidney may show more severe arteriolosclerosis, depending on severity of the HTN!
*Think the ischemic kidney w/ stenosis is essentially shut off from the blood supply, while the functional kidney is getting rocked by extremely high/persistent BP
The primary cause/inciting event of HUS differs from TTP how as far as pathogenesis?
- HUS = caused by endothelialinjuryandactivation
- TTP = inciting event is platelet activation –> aggregation

What is the trigger for endothelial injury in typical HUS vs. atypical HUS?
- Typical HUS - trigger is Shiga-like toxin (EHEC > Shigella)
- Atypical HUS - trigger is excessive activation of complement
What are the 3 major findings in the Thrombotic Microangiopathies (HUS and TTP)?
- Thrombi in capillaries and arterioles
- Microangiopathic hemolytic anemia
- Thrombocytopenia**** (big clue in a question stem!)
Who is most often affected by Typical HUS?
How is this form treated/managed and prognosis?
- Mainly children
- Renal failure is managed w/ dialysis and most pts recover normal renal function within weeks
- Long-term prognosis is variable due to renal damage
What are the 2 common inherited deficiencies which cause Atypical HUS?
- Deficiencies in complement regulatory proteins
- Most common = Factor H (breaks down alternative path)
- Some are deficient in Factor I and CD46
Other than inherited deficiencies of complement, what are some of the other causes of Atypical HUS?
- Antiphospholipid syndrome, either 1° or 2° to SLE
- Pregnancy –> postpartum renal failure
- Vascular diseases of kidney: systemic sclerosis and malignant HTN
- Chemotherapeutic and immunosuppressive drugs
- Irradiation of kidney
What is the prognosis of Atypical HUS?
Generally not as well as typical due to underlying conditions
Thrombotic Thrombocytopenic Purpura (TTP) is classically manifested by what pentad, what is the dominant feature?
1) Fever
2) Neurological sx’s = Dominant feature
3) Microangiopathic hemolytic anemia
4) Thrombocytopenia
5) Renal failure
TTP and Atypical HUS both appear more commonly in adults, occassionally having similar sx’s. How are they distinguished from one another?
Presence of normal ADAMTS13 in plasma = Atypical HUS
TTP is associated with inherited or acquired deficiencies in what?
The most common cause is due to what?
- ADAMTS13 = plasma metalloprotease which regulates vWF
- Inhibitory autoantibodies against ADAMTS13 = MOST COMMON!
Who is most often affected by TTP and it typically presents before what age?
- Woman
- Presents before 40 yo
What is the standard of treatment of TTP?
Plasma exchange to remove autoantibodies + provide functional ADAMTS13
In TTP, deficiencies of ADAMTS13, a negative regulator of vWF, permits the formation of what?
Abnormally large multimers of vWF that activate platelets
Light microscopy of chronic disease associated with atypical HUS/TTP will show what?
- Mildy HYPERcellular glomeruli
- Thickened capillary walls
- Splitting/reduplication of BM (“tram-tracks”)
- “Onion-skinning” of arterial walls
What are the morphological characteristics seen on micrcoscopy in both HUS/TTP?
Which arteries will show necrosis? (quiz question!)
- In acute, active dz, the kidney shows patchy or diffuse CORTICAL necrosis and subscapular petechiae
- Thrombi occluded glomerular capillaries
- Mesangiolysis
- Interlobular arteries w/ fibrinoid necrosis of wall and occlusive thrombi
Bilateral renal artery disease (aka atherosclerotic ischemic renal disease) is a common cause of what in older individuals?
Chronic ischemia w/ renal insufficiency, sometimes w/o HTN
How is bilateral renal artery disease definitively diagnosed?
Treatment?
- Arteriography
- Surgical revascularization
Atheroembolic renal disease is caused by what?
Most often seen in whom and when?
- Fragments of atheromatous plaques from aorta or renal artery embolize into intrarenal vessels
- Most commonly in older adults w/ severe atherosclerosis, esp. following surgery on AA, aortography, or intra-aortic cannulization

The emboli of Atheroembolic Renal Disease can be identified in the lumens of arcuate and interlobular arteries due to what appearance?
Content of cholesterol crystals, which appear as rhomboid clefts

Sickle-cell disease (homozygous) or trait (heterozygous) may lead to sickle-cell nephropathy, which is most commonly manifested how?
On occasion can develop into what serious disorder?
- Commonly, hematuria and hyposthenuria (inability to concentrate)
- Patchy papillary necrosis may also occur
- Proteinuria is also common, sub-nephrotic range (<3.5 g)
- On occasions, overt nephrotic syndrome arises –> FSGS

Diffuse Cortical Necrosis is an uncommon condition occuring most frequently after what?
Appears how morphologically?
- Obstetric emergencies, septic shock, or extensive surgeries
- Coagulative necrosis of both glomeruli and tubules, with necrosis confined to cortex

Why are the kidneys a common site for the development of infarcts?
Most infarcts are due to?
- Receive 1/4 of the cardiac output, “end-organ” vascular supply, and lack of collateral circulation
- Most infarcts are due to embolism

What are the most common source of emboli leading to renal infarcts?
- Mural thrombosis from left atrium/ventricle due to MI
- Vegetative endocarditis
- Aortic aneurysms
- Aortic atherosclerosis
Due to the lack of collateral blood supply, how do renal infarcts appear morphologically?
Shape?
- Sharply demarcated,pale, yellow-whiteareas ofcoagulative necrosis
- Wedge-shaped



