ch18 Flashcards
Rutherford experiment disproved which model for the atom?
describe the model
The Thompson model or plum pudding model
atoms were a plum pudding with electrons embedded throughout a positive sphere
how do you investigate the structure of an atom
with scattering experiments
particles are accelerated to a high energy and directed at a target
Describe Rutherford’s experiment and his results
A stream of alpha particles from a radioactive source was fired at a very thin gold foil.
The angles at which the particles were scattered were recorded
some passed straight through (so most atom is empty space)
but a few of the alpha particles bounced right back from the foil being scattered at angles greater than 90 degrees
What could Rutherford conclude after his experiment?
The atom is mainly empty space.
the core must be massive on an atomic scale to deflect alpha particles through large angles, but its much smaller than an atom as very few particles deflected through more than 90 degrees
alpha particles deflected due to electric repulsion between positively charged alpha particles and positive core in gold atoms.
The centre of the atom must have a large, positive charge. Rutherford named this the nucleus.
The nucleus must be tiny, but massive (mass).

1 in how many alpha particles were turned back and what does this suggest about the size of a nucleus
diameter of atom
diameter of nucleus
1 in 10000 turned around so nucleus is 10 times smaller than the atom
diameter of atom is 10-10m
diameter of nucleus therefore is 10-14 m

what happens to the deflection angle if the alpha particles are slowed done
how is scattering affected if the nuclei has less electric charge
relationship between scatter angle and inverse square law
if alpha particles are slowed down more would be deflected at greater angles since the nucleus would be able to turn them back more easily
nuclei of smaller electric charge scatter alpha particles less strongly
the pattern of number of alpha particles scattered at different angles fits the pattern expected from the inverse square law for electric repulsion

alpha particles are highly ionising and so will lose their energy over a short distance ionising air particles
by evacuating the apparatus (removing air particles) you cen minimise collisions and therefore get an accurate measure of the deflection angles
kinetic energy of alpha particle at its closest approach
0 kinetic energy
what is electrical potential energy equal to at a large distance from the nucleus
at a large distance from the nucleus, alpha particle electrical potential energy is equal to its initial kinetic energy
equation for initial kinetic energy
charge on alpha particle
charge on gold particle
initial kinetic energy = electrical potential energy = kQq / r
alpha: 2e = 2 *(1.6*10-19)
gold: 79e = 79 * (1.6*10-19)

force that a particle experiences near a nucleus
F = KQq / r²

What did Rutherford discover about charge at the centre of the atom?
Some of the alpha particles were deflected through large angles, so the centre of the atom must have a large positive charge to repel them.
What value do you need when you are estimating the distance of closest approach of an alpha particle that has been fired at a gold nucleus?
The alpha particles initial kinetic energy


What value do you need to find the charge of the nucleus?
The atom‘s proton number, Z
how are electrons similar to alpha particles
electrons are scattered by the nucleus in the same way alpha particles are, but the force is attractive this time.
how to produce high energy electrons
using particle accelerators
one application of particle accelerators
dental x-ray tubes are particle accelerators
electrons boiled off heated wire and accelerated towards positive target
describe the change in energy for electrons in dental x-ray tubes
rearrange to give velocity
electrons lose electrical potential energy (qV) and gain kinetic energy (1/2mv²)
qV = 1/2mv²
can rearrange for v (velocity)
V = accel voltage
q = charge
m = mass of electron
limitations of momentum equation
accurate at low speeds but not at high speeds
when v << c
einstein momentum equation
momentum = relativistic factor * mass * velocity
total energy of free particle
kinetic energy + rest energy
= relativistic factor * m * c²
equation for energy when particle at rest
relativistic factor is 1 at rest so
Erest = mc²
what is rest energy equivalent to
mass
Erest = m
equation to calc relativistic factor using energy
rel factor = Etotal / Erest
ymc² / mc²
what is a linear accelerator and how does it work
a long straight tube containing electrodes which change charge signs
electrons are acclerated between a pair of electrodes which have an alternating current applied to them
its timed so that the electron is always attracted to the next electrode and repelled from the previous one
length of electrodes increases because electrons are getting faster so they must stay in the tube for the same amount of time so that electrodes pd doesn’t change too early
the max speed of the electrons is only limited by the length of the accelerator, so they can approach the speed of light
momentum approximation equation
wavelength equation using this
when v is similar to c we can say momentum = y * m * v goes to momentum = y * m * c
Etotal = y * m * c²
so
p = Etotal / c
λ = h / p
λ = h * c / Etotal
sin theta equation involving nuclear diameter
sin theta = 1.22(wavelength) / nuclear diameter
can get wavelength from hc / E
what are quarks
quarks are the building blocks for hadrons (protons and neutrons)
how are quarks used to make protons and neutrons
to make the nucleons you need the 2 types of quark, the up quark (u) and the down quark (d)
the up quark has charge 2/3
the down quark has charge -1/3
so proton = up(2/3) + up (2/3) + down (-1/3) = +1e
neutron = ddu = -1/3 - 1/3 + 2/3

how do you use quarks for antiprotons and antineutrons
an antiproton is a p with a line above it
antineutron is a n with a line above it
to make these you need antiquarks, quarks with the opposite charge
these are the anti-up (-2/3) and anti-down (1/3)
pic shows how to make them

what holds quarks together inside a particle and how does it work
gluons are particles that are very strong and hold quarks together
quarks attract one another by exchanging gluons
gluons hold protons and neutrons in one piece keeping the nucleus together
this interaction is known as the strong interaction
antiparticle of electron
how do antimatter differ from matter
the antiparticle equivalent of electron is the positron
all antiparticles have the same mass as particles just the opposite charge
2 ways to represent an alpha particle
42α
42He
what are leptons
what do they do
example
leptons are fundamental particles that interact through a nuclear force called the weak interaction
this weak interaction is reponsible for the beta decay
electrons and neutrinos are leptons
what are hadrons
what do they do
examples
hadrons are composite particles made up of quarks
they interact through the strong interaction
protons and neutrons are hadrons
what are neutrinos
neutrinos are particles that you can assume to have 0 charge and 0 mass
how are positrons emitted from a nucleus
what happens after its been emitted
what happens to the energy
in a process called beta decay (B+)
a positron cannot exist in the world, almost instantly it encounters the outer electron of another particle and the 2 particles annihilate each other
matter is destroyed, but energy can’t be so all the mass of the particles gets converted to energy and is carried away by gamma rays travelling in opposite directions

what is conserved during an annihilation event
electric charge
linear and angular momentum
energy
lepton number

lepton number for electron and positron
lepton number for electron: 1
lepton number for positron: -1
what is pair creation
what happens in it
requirements
the opposite of annihilation
creation of equal amounts of positron and electron by gamma ray photon of sufficient energy near a massive nucleus
gamma ray photon must have energy equal or more to 2mc² (total rest energy of electron and positron) which is 1.022MeV
why do you need a nucleus near a gamma photon to create an electron positron pair
a 1.022MeV gamma photon has enough energy to create an electron-positron pair but it can’t create this in free space
this is because the photon has momentum p = E / c and momentum must be conserved
pair creation can occur near a nucleus which then carries away some momentum so that both energy and momentum is conserved
lepton number and electric charge are conserved because the remain at 0

how to find max lambda of gamma ray photon after electron and positron made from single gamma ray photon
E = 2mc²
then E = hc / lambda
rearrange and then calc for lambda
what does a nucleus do when it has too many neutrons
what is this process called
the nucleus has too many neutrons so it can decay by emitting an electron
neutrins are unstable and decay into a proton
this is beta minus decay (B-)
n -> p + e + antineutrino
what is an issue with beta minus decay and what did it lead to the discovery of
use the decay of strontium 90 to ytrrium 90
equation
9038Sr -> 9039Y + 0-1e
neutron turns into proton to keep charge conserved and all numbers conserved
electron (lepton) on right but no lepton (electron) on left
difference in rest energy between Sr and Y but every beta particle doesn’t have same energy as the difference
so it was suggested that theres another particle emitted carrying the extra energy, no charge, small mass and weak interactions
this is the neutrino (00v)
so when the Sr decays to Y a beta particle is emitted along with an antineutrino (same as neutrino with a line above v)
equation: 9038Sr -> 9039Y + 0-1e + (00v)line above v
why is beta- decay not due to the strong interaction
n -> p + e- + antineutrino
electrons and v are leptons
leptons are not affected by strong interaction, the decay can’t be due to the strong interaction
how are neon light signs evidence that electrons in atoms have discrete energy levels
their light comes in sharp discrete spectral lines
how to find energy of photons given energy of 2 discrete energy levels
the energies of the photons correspond to the difference in energy levels in the atom
describe the franck and hertz experiment
F and H experiment gives evidence for discrete energy levels
tube filled with gas atoms that collide with electrons as they travel along the tube
filament on left of tube is heated so electrons ‘boiled off’ and accelerated towards the wire grid at a certain positive potential. the anode will be at a slightly lower potential than the grid
the difference in potential (e.g. 0.2V) is the minimum kinetic energy in electronvolts that electrons need to reach the anode (0.2eV)
the current registered on the sensitive ammeter is a measure of the number of electrons passing through it each second
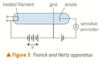
fh exp:
describe and explain the graph and what it gives evidence for

describe: increasing accelerating pd increases current but at Vc,2Vc and 3Vc current falls then rises
exp: at low accelerating pds the electrons from the filament make elastic collisions with gas atoms bouncing off without loss of energy
increasing pd increases the current registered on sensitive anode
but at Vc, accel electrons have enough energy to knock an electron in the gas atom to a higher energy level, so some of the accel electron energy is given to the gas atom in an inelastic collision. this means some electrons don’t have enough energy to reach the anode so current falls
at 2 and 3Vc accel electrons can give energy to 2 or 3 atoms
drops in current show inelastic collisions can only happen at specific electron energies. this shows that there are specific energy levels in the atom which the electrons are fixed to

are electrons only allowed to have cerain energies
not really, because electrons have de broglie wavelengths associated with them
atoms can be thought of having a potential energy well in which electrons are bound

explain the standing wave for electrons with equation for energy of electrons
as electrons are trapped in the potential energy well of the positive nucleus
standing waves for electrons can form
de broglie wavelengths are limited by the size of the box
we know p = h / lambda
energy of electron is small so use non relativistic expression Ek = 1/2mv² = p² / 2m
Ek = h² / 2mλ²
how does the size of the box trapping the electron affect wavelength and energy and momentum
how does it affect the fixed energy values
the smaller the box trapping the electron, the smaller the wavelength and so the greater the momentum and kinetic energy
the size of the box determines the wavelength and so the allowed energy values
hydrogen atom model for standing waves
applying model to hydrogen with a single electron with no potential wells but instead hard walls a fixed distance apart
what does energy of electrons depend on in the hydrogen atom standing wave model
what does this produce
the width of the box and the number n of half-wave loops in the standing waves
produces a number of alowed energy levels labelled by a quantum number n at the ground state
charge, and lepton number of
proton, antiproton
neutron, antineutron
electron, positron
neutrino, antineutrino

how much energy do electrons need to escape the potential well in the wave model of the atom
has to have a total energy (Ek + Ep) of greater than 0
using the de broglie wavelength equation how many wavelengths are there in n = 1 and 2 of the electron standing wave model
lambda = h / p = h / mv
n = 1, half a wavelength between walls. lambda = 2d
n = 2, one complete wavelength between walls, lambda = d
n = 3, 2/3d

force at right angles to direction of motion

describe the process for annihilation in Positron Emission Tomography and then for brain tumour detecting
isotope of O2 gives off positron which interacts with outer electron of another atom, both destroyed in annihilation event, energy taken by gamma ray photons
in brain tumours isotope of O2 injected into blood that gives out positrons while travelling throughout the body but a tumour will have a rich supply of blood so lots of decay there gives lots of gamma ray photons which leave head and are detected
why does the graph look like this

electrons can show wave like properties
the red line is if electrons act like the particles that they are
the blue line is the actual scattering due to electron diffraction showing the ‘kinks’ in the curve
electrons follow rutherford’s scattering but the curve superimposed on it is the diffraction pattern because electrons act in a wave like manner
if given graph similar to this how do I work out nuclear diameter of particles
look for lowest kink on diagram and read at what angle that is on the graph
nuclear diameter = 1.22lambda / sin theta
lambda = hc / E
describe bubble chambers and what they do
in bubble chambers fast particles ionise water vapour creating a trail
there are 2 trails due to the charge on the particle
one due to +ve charge and the other -ve charge
inwards spiralling because they’re losing energy
kinetic energy for electron using de broglie wavelength

as only certain wavelengths can fit in an electron how is ke affected
only certain wavelengths of electrons can fit inside atoms at a certain distance from nucleus ke is also limited to certain energies
what does En = -13.6eV / n show
find out En when n = 1 and 2
this shows the energy required for energy levels for hydrogen where n is the energy level so n = 1 at ground state
when n = 1, En = -13.6eV / 1² = -13.6eV
when n = 2, En = -13.6eV / 2² = -3.4eV
what happens when an electron goes from a higher energy orbit to a lower energy orbit
electric potential energy gets more negative
the change in energy is the energy of the photon emitted
find the wavelength of the photon emitted when an electron falls from n = 3 to 1

n = 3 = -1.5eV
n = 1 = -13.6eV
change in energy = 12.5eV * 1.6 * 10-19 = 1.936 * 10-18J photon energy E
E = hc / lambda
lambda = hc / E = 1 * 10-7m
visible light = 400Nm to 700Nm
this is below it so its in the UV range
this is correct as drop from n = 3 to n = 1 is in the lyman series
why is an electron better for diffraction compared to alpha particles
electrons aren’t affected by strong nuclear force so they can touch the nucleus and turn around whereas alpha particles would be captured by gluons and absorbed into the nucleus
quick way to calculate velocity with a known value of relativistic factor
v / c = ROOT(1 - 1 / (rel factor)²)


