Cell Cycle/Cancer (D.M) Flashcards
What are the two main types of cell death?
Apoptosis –> opposite to cell proliferation
- Programmed cell death –> apoptosis –> A lot more regulated –> contents that need to be broken down are placed in lipid-bound vesicles which are then digested by phagocytes.
- Necrosis –> cells basically explode –> contents of the cell are released –> not useful as its a waster plus it may be harmful –> often results in inflammation.
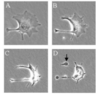
Describe what an apoptotic cell would look like during different stages of apoptosis.
A –> cell provided with apoptotic stimulus
B –> shrinks
C –> shrinks further
D –> vesicle formation
Is apoptosis a normal process?
Apoptosis –> normal process
During development, it plays an essential role –> for example tissue between fingers removed via apoptosis + blood vessels are solid –> centre removed via apoptosis + neurons removed from the brain (connected in the wrong way)
Also important during adulthood –> cell damaged too much? –> cell dies via apoptosis/T cell elimination

What are the different stages in the cell cycle?
- Interphase –> majority of the time in this stage (G1 phase, S phase and G2 phase)
- Prophase –> chromosomes become visible
- Metaphase –> align on the central equator + spindle formation
- Anaphase –> separation –> move to opposite ends
- Telophase –> separation into two cells.
- Cytokinesis
2-6 –> Mitosis (division process)

What happens in G1, S and G2 phase?
Interphase
G1 –> Sensing phase –> good conditions for division?
S –> Replicates DNA
G2 –> Checking and cell gathers energy
G –> Growth or Gap
What happens in S phase?
DNA replication –> end up with two sister chromatids which will come together later in prophase to form a chromosome. Both sister chromatids are held together by cohesin complexes at the centromere which is a constriction point.

What happens in prophase?
Prophase
- Chromosome condensation –> important because the average length of DNA in chromosome about 5cm – nuclear diameter 5um (0.005mm).
How doe this happen?
Naked DNA –> wrap it around nucleosomes (beads on a string arrangement) –> wrap the nucleosomes around each other to form chromatin fibre –> loop the fibres in a protein scaffold –> associate scaffolds –> forms chromosomes.
- Mitotic spindle starts to form –> co-ordinated by centrosomes (type of organelle –> allow microtubules to grow from a specific position)

Structure of the spindle?
Microtubules originate from the centrosome
- Microtubules that move away from the centre –> called astral
- Microtubules that overlap with each other –> called overlap microtubules
- Microtubules that attached to chromosomes –> called kinetochore microtubules
What happens in metaphase?
In ProMetaphase
- Chromosome fully condensed
- Centrosomes at opposite ends of the cell
- Spindle formation is mainly complete
- Nuclear envelope breakdown (one of the main changes)
In Metaphase
- Chromosomes align along the equator –> achieved using spindle fibres that push and pull the chromosomes –> microtubules attach to kinetochore (the protein complex assembled at the centromere that binds to microtubules of the spindle) -> balanced pushing and pulling leads to central alignment.

What happens in anaphase?
Anaphase
- Sister chromatids separate –> allows chromatids to move opposite sides
- There are a bunch of cohesin proteins that hold the two sister chromatids together –> proteolytic enzymes needed –> three key players: APC (anaphase-promoting complex, Securin (inhibitor of separase), separase (protease –> specific for cohesins).
1. When chromosomes are aligned along equator –> APC is activated.
2. Cleaves securin –> releases active separase
3. Active separase can now cleave the cohesin –> sister chromatids are no longer attached.
4. No force holding chromatids together –> pulling action of microtubules pulls chromatids to opposite poles.

Explain what happens to the microtubules when the chromosomes separate.
Microtubules can be found in two forms:
- Growing –> has a GTP cap promotes elongation
- Shrinking –> GTP cap no longer present –> dynamic instability –> allows the microtubules to shrink rapidly.
Hence –> when chromosomes need to be separated –> Cap is removed –> shrinkage of microtubules –> seperation.
Three different types of motion that occur
- Kinetochore microtubules –> dynamic instability –> pull sister chromatids
- Overlap microtubules –> Push against each other –> push away centrosomes –> pushes centrosomes to opposite ends of the cell
- Astral microtubules –> Pulling from the edge of the cell –> further separating microtubules.

Explain what happens in telophase.
Telophase
- Chromosomes at opposite poles must decondense
- Nuclear envelope forms
- Microtubules/Spindle breakdown
What happens in cytokinesis?
Formation of two cells
- Twisting cells –> forms constriction point –> cells pop apart –> actin ring contracts separating the membranes

How are organelles distributed?
- When organelles are really abundant –> stochastic/random process
- Only one of the organelle –> active distribution of organelle –> undergo their own division
What experiments were conducted on cdc mutants?
Yeast were given mutations which resulted in…
- Yeast cells that grew too large before dividing –> resulted in elongated yeast.
- Yeast cells that didn’t grow much before dividing –> resulted in small circular yeast.
They then figured out the what mutations the yeast cells had –> complementation analysis –> From this analysis they figured out that there were 60 different genes that regulated the cell proliferation cycle (varying amounts of importance –> fundamental and accessory)
What was one of the main genes involved in cell cycle regulation?
Gene called cdc2 –> codes for a protein kinase (involved in signalling –> phosphorylation) –> one of the key regulators of proliferation process.
cdc2 can phosphorylate serine and threonine side chains on other proteins –> essential for cell cycle –> without you get elongated cells.
cdc2 concentration remains constant, however, its activity level fluctuates.

What are cyclins and how does their concentration differ along cell cycle?
Cyclin is a family of proteins that control the progression of cells through the cell cycle by activating cyclin-dependent kinase (CDK) enzymes.

How does CDK and cyclin activity relate?
CDK and cyclins activity similar activity levels –> maximum activity in G2 phase.
This is because the cyclin is a regulatory subunit for CDK (same as cdc2 but for humans) –> no cyclin –> kinase is not activated –> no activity.
How many different cyclins are there? How do they function?
There is a cyclin equivalent for each stage of the cell cycle –> they tell that specific phase to progress.
Basically –> specific cyclin is made at a particular time –> activates the CDK –> initiates a particular step in the cell cycle –> cyclin is destroyed at a defined time –> stops action CDK (step cannot be repeated) –> allows for ordered progression of cycle.
Note
G2 is the regulatory step for yeast
However, in humans, G1 is the regulatory step.

What is the restriction point?
Cell passes restriction point –> it must go through the entire cell cycle –> occurs in late G1 phase.
What is a key protein in restriction point control?
Retinoblastoma protein (Rb) –> 1 05kDa
Two forms of the Retinoblastoma protein –> Hypo- (active) and hyper-phosphorylated (inactive) form –> Low and high phosphate content respectively –> the phosphorylated forms have different conformations
G1 –> Hypophosphorylated form
S/G2/M –> Hypophosphorylated and Hyperphosphorylated form
Phosphorylation is cyclical
Note –> RB can be phosphorylated by G1 cyclins/CDKs

Explain the different phosphorylated forms of Rb effect the cell cycle.
Model of Cell cycle
Rb –> stops hyperproliferation (division)
G1 –> hypophosphorylated –> active form –> inhibits continuation in cell cycle
S/G2/M –> hyperphosphorylated form –> Rb is not stopping hyperproliferation –> allows for the latter stages in cell cycle.
How does Rb specifically influences the cell cycle?
When Rb is in the hypophosphorylated form…
Rb interacts with E2F protein –> transcription factors that bind to DNA in a site-specific manner –> by binding to E2F –> it can not act as a transcription factor.
What gene expression are impacted by E2F?
- S-phase cyclin
- DNA polymerase
- DHFR (metabolic gene –> responsible of nucleotide production for DNA replication)
- etc….
What happens step by step to Rb when a cell decides to go through the division process?
- G1 cyclins are activated
- Cyclins bind to the cdks
- Phosphorylates the Rb protein –> Hypo to Hyper phosphorylated form
- It undergoes a conformational change —> no longer bonded to E2F.
- E2F is free –> Activates transcription of genes required for S-phase
- The cell is now committed to S-phase
Once this occurs –> there is no going back –> irreversible switch.























