CBIO 5: Cancer Immunobiology Flashcards
Observe the learning outcomes of this session

What is the fundamental of the professional antigen-presenting cells?
Give an example of them
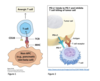
- dendritic cells: act as a bridge between innate and adaptive immune system
- They patrol the body in search of antigens that they present to T cells by displaying them on a complex of molecules called the Major Histocompatibility Complex (MHC) or Human Leucocyte Antigen (HLA).
- They serve a fundamental function - in fact, T cells are not able to detect antigens on their own and require the T cell receptor (TCR) to recognise antigens displayed on MCH
What is an antigen?
- An antigen is a substance able to produce an immune response
- Antigens are usually peptides.
What are the three types of tumour cell antigens?
Briefly describe them
- tumour specific antigens (TSA):
- Tumour specific antigens are present only in cancer cells and normally they are the product of a genetic aberration.
- An aberrant gene can be still transcribed and translated and the protein produced will be new to the immune system.
- tumour-associated antigens (TAA):
- As you have already learned cancer cells can increase the expression of some proteins that make them thrive, for example, some growth factor receptors
- The immune system is tolerant to these proteins because they are also present in normal cells.
- However, some TAAs are expressed at a very low level in normal cells and when expressed in higher levels within cancer cells they can trigger an immune response.
- They can also be used to develop some immune therapies.
- oncofetal antigens (TAA):
- Some proteins are expressed during early embryonic development and are switched off in adult life.
- These proteins are expressed before the immune system fully develops and acquires self-tolerance.
- Cancer cells have the ability to activate the transcription and translation of embryonic genes that make them thrive.
- Once these are active, the immune system encounters them for the first time and therefore is able to mount a response.

Review the main characteristics of tumour antigens
- examples of antigen type
- approved immunotherapies for target antigen

Look at the table
There is another group of antigens described in the table (Cancer/testis antigen). Read the description and select which category you think it most closely resembles:
- TSA
- TAA

- TAA
- because they are expressed by various tumour types but nevertheless are also expressed in normal tissues (in reproductive tissues
- so they are not only specific in tumour cells
Which antigens would you consider safe to target and why?
- TSAs
- TAAs

- TSAs
- since they are only expressed on tumour cells
- TAAs are useful targets too
- e.g. ErbB2 is successfully targeted in breast cancer
Find which of these antigens are expressed in cancer but not in normal cells?
- MAGE-A3
- Claudin3
- HER2
- HPV E6 and E7

- HPV E6 and E7
What is immune surveillance?
- this is the stage when effectors cells play a fundamental role in the immune response to tumours
- they are capable of mounting a response that can effectively kill cancer cells
What are some cells involved in immune surveillance?
- antigen presenting cells (APCs)
- helper T cells (THs)
- cytotoxic T lymphocytes (CTLs)
- natural killer cells (NK cells)
- B cells
- inflammatory macrophages (M1)
What are the two types of antigen-presenting cells?
- professional antigen presenting cells
- non-professional antigen presenting cells
Describe professional antigen-presenting cells
Give examples of them
How do they detect tumour antigens?
- These cells are very efficient at internalising and processing external antigens by phagocytosis.
- The resulting peptides are loaded into the Major Histocompatibility Complex II (MHC II) and displayed on the cell membrane.
- Dendritic cells (DC), macrophages and B cells are all professional antigen-presenting cells.
- Antigen-presenting cells may detect tumour antigen shed by tumour cells or they can sample fragments of tumour cells in and around the tumour microenvironment.
Describe non-professional antigen-presenting cells
Give examples of them
How do they detect tumour antigens?
- These are all the nucleated cells in our body including antigen-presenting cells and they present self-antigens (both normal and mutated), or antigens that end up in the cytoplasm, for example, from viruses.
- These antigens are processed through the proteasome and loaded into the Major Histocompatibility Complex I (MHC I) in the endoplasmic reticulum and then displayed at the cell surface.
- As explained earlier when a normal self-antigen is displayed on MCH class I, the immune system does not mount a response, but when this is a mutated self-antigen or viral antigen it does.
Describe how antigen-presenting cells activate T-cells
- The first signal required for T cells to attack cancer cells is an interaction between the MHC I/II and the T cell receptor (TCR).
- The second signal is the interaction between co-stimulatory molecules present on the surface membrane of T cells and APCs.
- A well-known example is the interaction between CD28, which is present on T cells, and CD80 and CD86 which are expressed on APCs.
- APCs release specific subsets of cytokines that provide further information to T cells allowing them to mount the most appropriate response.

Describe immune checkpoints when it comes to T-cell activation
Why?
- T cell activation requires several switches because improper activation of T cells can have serious consequences.
- As such, once the immune response is completed, T cells need to return to a resting state.
- Alongside an activation mechanism, there is a mechanism that acts as a break so that the immune response is controlled.
- Examples of immune checkpoint receptors are cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed death-1 (PD-1).
- When these are engaged with their respective ligands, CD80/86 and PD-L1/2, they send an “off” signal that places T cells at rest.

Describe the ligand binding and affinity in this diagram for T-cell activation

- CD28 and CTLA4 share the same ligands CD80/86.
- However, the affinity of CTLA4 for CD80/86 is far superior to that of CD28.
What are some of the functions of helper T cells?
- activate B cells to produce antibodies
- Induce better killing ability in macrophages
- Dampen the immune response when tolerance is required
- Help in the activation of cytotoxic T cells.
Why is it that only professional antigen-presenting cells can present to helper T-cells?
- Helper T cells express CD4 on their surface and they are called CD4+ cells.
- CD4 is a co-receptor of the TCR that allows the recognition of antigens presented via MHC class II, as illustrated in the figure below.
- Therefore only professional antigen-presenting cells can present to helper T cells.

Describe cytotoxic T lymphocytes
What antigens do they recognise?
- Cytotoxic T lymphocytes (CTLs) are professional killers.
- When they identify cells expressing viral or tumour antigens they release granules filled with toxic substances to kill the target cells.
- Potentially any cell in our body can become cancerous or infected by viruses and therefore antigen presentation to CTLs has to occur from all these cells.
- For this reason, CTLs recognise antigen presented via MHC class I, which is expressed by all nucleated cells.
- CTLs express the TCR co-receptor, CD8 that allows the specific recognition of antigens presented via MHC class I as illustrated in the figure below.

Study this diagram of CTLs in action when dealing with a tumour cell

Describe natural killer cells
- response in cancer cells
- activation
- mechanisms
- Natural killer cells (NK cells) are part of innate immunity.
- They are at rest when they detect inhibitory ligands that are superior to the activating ligands on the surface of target cells.
- MHC class I for example is an inhibitory ligand.
- However, viral infection including that with onco-viruses can downregulate the expression of MCH class I.
- Similarly, cancer cells with high mutation rates may produce aberrant MHC class I.
- As consequence, NK cells receptors are mostly engaged with activating ligands.
- Another cause of activation is the expression of stress signals.
- These are common in cancer cells and are caused by stressors such as DNA damage.
- When the stress/activating ligands are detected, NK cells become active and kill the target cells.
- Their killing mechanisms include the production of cytokines and cytotoxic mediators which induce apoptosis in the target cell.
- Have a look at the picture below that summarises the conditions for NK cell activation.

Describe B cells and antibodies in the framework of cancer
- B cells produce antibodies against tumour antigens.
- Antibodies in the context of cancer have two fundamental roles: one role is that they can block the interaction of a tumour antigen (for example a receptor) with a ligand.
- However, the most important function is tagging.
- As we will see later cancer cells tagged with antibodies can be identified and destroyed by other cells of the immune system
Describe macrophages
- functions
- Macrophages are cells of the innate immunity and professional antigen-presenting cells.
- They serve an important function to eliminate pathogens and cell debris by phagocytosis.
- However, in the immune response to cancer, they have another important function:
- the antibody-dependent cell-mediated cytotoxicity (ADCC).
- Macrophages express the Fc receptor which is able to bind the Fc portion of an antibody when this is bound to a tumour antigen.
- Macrophages then release lytic enzymes, reactive oxygen and nitrogen species, and anti-tumour cytokines such as tumour necrosis alpha (TNF-a), as shown in the figure below.

What other cells apart from macrophages express Fc receptors and are capable of ADCC?
- NK cells