Cardio Path Flashcards
(60 cards)



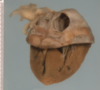
Organising anteroseptal transmural myocardial infarct with biventricular dilatation and thrombosis

Lines of Zahn, organisation of thrombus
Fate of thrombus
- Resolution/dissolution
- Emboli formation
- Organisation and recannalisation (return of blood flow)
- Propogation
Anti-mortum vs post-mortum clot
Antimortum:
- lines of zahn
- attached to vessel wall
- firm but frailable
Postmortum:
- No lines of zahn
- not attached to vessel wall
- rubbery, gelatinous

Saddle embolism

Pulmonary embolism and infarction with fibrinous pleurisy

DVT
Reperfusion injury
blood and O2 –> free radicals and ROS –> increased inflammatory response
signs of coagulative necrosis on histology
- no nuclei
- retention of cellular architechture
- eosinophilic cytoplasm
What stage of myocardial infarct?

Swelling and increased opacity of tissue due to coagulative necrosis
What stage of MI?

Inflammatory response to area of infarction
What stage of MI?

Ingrowth of granulation tissue
What stage of MI?

Formation of mature scar tissue

Severe myocardial ischaemia with multiple foci of old and recent infarction. Cardiac hypertrophy and dilatation.

Old and recent myocardial infarction with extensive left ventricular mural thrombosis. Note the pericarditis associated with the recent infarct, and the lines of Zahn in the thrombus.

Hypertrophy, rupture of papillary muscle

Concentric hypertrophy

Atherosclerosis - cholesterol clefts, fibrous cap, foam cells, lipid deposits

Hyperplastic arterioloscerosis due to malignant hypertension

Hyaline arterioloscerosis due to HTN or DM

Monkeburg arterioloscerosis - calcification in media due to ageing

Atherosclerotic plaque complicated by the formation of an occlusive thrombosis in the vessel. The thrombosis is reorganising. It is likely to be due to rupture of the plaque.

Fusiform aneurysm and mural thrombus