Cardio and renal L1 Flashcards
end stage renal disease is a condition closely related to ..
end stage renal disease is a condition closely related to high blood pressure
Label + 1-4?

0: Rapid depolarisation = NaV
1: Ca2+ current
2: prolongued plateu phase
3: K+ open - repolarisation
4: resting

The fast depolarising phase of the cardiac action potential is due to the opening of ….
The fast depolarising phase of the cardiac action potential is due to the opening of sodium channels.
describe what it meant by inactivated NaC cahnnels?
open transiently - and wont open again until the membrane as repolarisaed
T or F
The difference is that in neurones and skeletal muscle the initial depolarisation needed to ‘gate’ the Na+ channels is produced by the action of a neurotransmitter, whereas in cardiac cells this depolarisation is provided by pacemaker cells
T
Describe the structure of the voltage gated sodium cahnnel
large central alpha subunit - flanked by a1 and a2
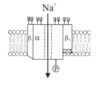
Like most membrane proteins the subunits need some phosphorylation and glycosylation to function correctly
P is a phosphorylation site and trident thingy are glycosylation sites.
T or f
In cardiac and skeletal muscle NaV channels have only b1 and a1 subunits
T
no B2 (previous card is NaV fromthe brain
The structure of the alpha subunit of the cardiac voltage-gated Na+ channel


what does the S4 segment fo the alpha NaV cahnnel do?
voltge sensor
NaV chanel:
The regions between the __ and __ form the extracellular entrance to the channel, while the __ segments line the channel pore.
The regions between the S5 and S6 segments form the extracellular entrance to the channel, while the S6 segments line the channel pore.
which domains in the alpha subunit of the NaV channel are important for producing inactivation?
cytoplasmic loop between domains III and IV is important in producing inactivation
can local anaesthetics be used to control cardiac dysrhythmias?
how come?
yes.
they act on the intracellular surface of the voltage gated channel and block it
what diameter aoxns are more sensitive to nerve blocks?
Broadly speaking, smaller diameter cells are more sensitive to the drugs than large diameter cells.
This means that the drugs will, at low concentrations, block nervous conduction while not affecting muscle action potentials.
Both sensory and motor fibres are affected by local anaesthetics.
In a mixed-fibre sensory nerve the sensation of pain is blocked first, followed sequentially by cold, warmth, touch and deep pressure.
This sequence corresponds to increasing diameters of nerve fibres.
what effect does anaesthetic have on the NaV channel?
enhances inactivation
why do local anaesthetics work best in smal diameter fibres?
because tey ajve a short space constnat
the scientist Hille did some experiments:
what did he conclude about the action of local anaesthetics?
- The local anaesthetics act on the intracellular surface of the voltage-gated Na+ channel.
- The drugs need to be uncharged to pass across the cell membrane - the more hydrophobic the drug, the better it is at crossing the membrane.
- The charged form of the drug is the form that interacts with the channel to produce the anaesthesia.
describe use dependance for local anaesthetics?
It is evidently necessary for the channel to be open for the majority of the anaesthetic effect to be initiated.
The more often the channel opens, the more likely it is that it will be blocked by the anaesthetic.
This phenomenon is known as ‘use- dependence’.
picture for lidocaine use dependance:
higher rate of firing = more anaesthesia

hwo does local anaesthetic gain access to the cell
diffuse across the cell membrane in their relatively hydrophobic, uncharged form.
is it the uncharged or charged form of the local anaesthetic which interacts with the intracellular receptor?
charged with the NaV receptor
whats going on here?

shows the effects of lidocaine

When some channels are left inactivated (in the right-hand panels) the inward currents are practically abolished by lidocaine, so it seems that the anaesthetic enhances the inactivation of the channels
whats going on here?

This Figure gives a measure of the proportion of Na+ channels that can be activated at any given resting potential.
In the presence of lidocaine or benzocaine this inactivation curve is shifted to the left - more negative prepulse values. Thus, in the presence of lidocaine and benzocaine the proportion of channels that are in a state which cannot be activated is increased at any given membrane potential.
describe tetrodotoxin
tetrodotoxin (or TTX) and saxitoxin (or STX)
They both are highly selective, reversible blockers of neuronal voltage-gated Na+channels
They do not affect voltage-dependence or inactivation.
which NaV channels is TTx more effective on?
TTX is considerably less effective on cardiac Na+ channels.
It is blocked only by micromolar concentrations of the toxin whilst the neuronal and skeletal muscle channels require only nanomolar quantities.
describe the Batrachotoxin
It acts on the intracellular portions of the channel to prevent inactivation and to move the activation potential to more negative potentials and so the channels open far more readily than in the absence of the toxin.
aconitine1 and veratridine act in similar ways and can provoke cardiac dysrhythmias.
Insect voltage-gated Na+ channels are similarly affected by the pyrethrin insecticides and DDT.
describe scorpion toxins
á- scorpion toxin is a polypeptide that acts from the outside of the voltage-gated Na+ channel. It inhibits inactivation and acts cooperatively with batrachotoxin to open the channel almost permanently. Its binding is voltage-dependent and is enhanced by batrachotoxin (which shifts the activation potential in any case).
do you know the strcutrue of the Ca2+ channel

do different Ca2+ channels in differnet tissues behave differently?
yep
The principal drugs acting on these channels are __________ (such as nifedipine), __________ (such as verapamil) and _____________ (such as diltiazem).
In current clinical practice, _______ and _______ are known as “non-dihydropyridines”.
The principal drugs acting on these channels are dihydropyridines (such as nifedipine), phenylalkylamines (such as verapamil) and benzothiazepines (such as diltiazem).
In current clinical practice, phenylalkylamines and benzothiazepines are known as “non-dihydropyridines”.
describe L-type Ca2+ channels?
- important in cardio vascular system
- sensitive to dihydropyridines - bind inactived channels but show no use dependance
- need large depol (+30mv) to open
- open for long time and inactivate slowly
- responseible for plateu phase cardiac AP
- activity modulation alters strength adn rate of contraction of the heart
- lrage single channel conductance
- Adr and NA enhance channel activity (throguh phosphorylation)
describe t-Ca2+ channels
- show rapid inactivation
- open transiently
- low single channel conductance
- not sensitive to dihydropyridines
- T-type channels occur together with L-type channels in nodal pacemaker tissues in the heart.
- Triggering of T-type channels can be sufficient to depolarise the cell sufficiently to activate L-type channels.
divalent cations can block whcih channels?
eg, Ni2+
block nonspecifically all ca2+ channels
describe photoaffinnity labelling?
very tough

where do Phenylalkylamines bind to calcium channels
S5 to S6 loop

where do Dihydropyridines bind to ca channels
- 2 binding sites
- segment S6 and part of its associated S5-S6 loop in domain III
- second is closely related to the phenylalkylamine binding site on the S5-S6 loop in domain IV

where do Benzothiazepines bind ca channels?
- 1 major drug - diltiazem
- block form outside
- bind in differnet place to dihydropyridines but modulate dihydropyridine binding.
- specific location unknown
If K+ channels open in a normal cell,… what happens?
If K+ channels open in a normal cell, current flows out of the cell, making the membrane potential more negative.
how do potassium channels contribute to the repolarisation after an AP
cardiac cells (like other excitable cells) possess voltage-gated K+ channels which open slowly on depolarisation (actually they start to open at the same time as the voltage-gated Na+ and Ca2+ channels) and the current they carry leads to the restoration of the membrane potential to its normal resting leve
T or F
K+ channels are ubiquitous, and do not only occur in excitable tissues
T
Nerve and muscle cells contain what types of K+ channels?
Nerve and muscle cells contain both voltage-gated and non-voltage-gated
K+ channels
T or f
K+ channels maintain the resting cell membrane potential in all animal cells
T
what determines the resting membrane potenital is atrial and ventricular cells?
the resting membrane potential in atrial and ventricular cells are determined by the resting membrane having a high permeability to K+ ions (i.e. dependent on the activity of K+ channels).
describe how the resting potential is set up?
the negative resting potential of atrial and ventricular cells is maintained by the Na+/K+ ATPase pump which exchanges three intracellular Na+ ions for two K+ ions.
The membrane at rest is highly permeable to K+ ions but to all intents and purposes impermeable to Na+ ions.
Due to the gradient of these two ions across the membrane, in combination with the different degree of permeability of the membrane to the two ions the resting potential (Vm . -80 mV) is close to the equilibrium potential for K+ (EK. -95 mV)
dual role of K+ channels in the heart?
- Repolarisation of the cell at the end of the action potential
- Stabilisation and modification of the resting cell membrane potential in the atria and ventricles
structural differnce between K+ and Na+ channels?
They have the six transmembrane segments familiar from Na+ channels, but only show a single group of the six segments, compared with the four of Na+ channels.
where is the voltage sensor in the K+ cahnnel;?
As in the Na+ channel, every third residue in the S4 segment is positively charged and mutagenesis experiments suggest that this forms the voltage sensor.
are all K+ channels voltage senssing?
no
Other K+ channels that do not display voltage-activation have a different structure
how many Kv proteins aggregate together to form a pore?
4
describe teh 2 types of inactivation of voltage gated K+ channels?
oltage-gated K+ channels show two types of inactivation.
- ‘N-type’ inactivation or ‘ball and chain inactivation’ - the N-terminus of the protein forms a ball which is ‘sucked’ into the pore, occluding it, as the result of electrostatic changes associated with depolarisation.
- ‘C-type’ inactivation, is slower and seems to be the result of movement of residues near the extracellular surface of the pore.
‘IK1 current’
what is this? - how does it affect cardio cells
the outeward K+ current flowing thorugh the largely open K+ channels maintaingin the resting potential.
usually doesnt really affect cells as theyre not depolarised for long enough for the cell to loose enough K+.
cardaic cells spend half their time depolarised, which leaves a large K+ gradient. so to stop K+ from flwoing out the cell in large quantities - theyre protected by the inward rectification current
describe Inward rectifying K+ channels
- called Kir channels
- some dont show voltage gating
- wide range
- regulated by phosphorylation, intracellular Ca2+ levels or by intracellular ATP
- not confied to excitable tissues
- Some channels show both voltage-gating and inward rectification
what causes the occulsion leading to rectification?
he rectification used to be thought to be due wholly to intracellular magnesium ions blocking outward current by lodging in the pore. However, it is now clear that besides magnesium, intracellular polyamines (especially the tetravalent polyamine spermine) are of great importance in accounting for the occlusion producing the rectification.
label the Kir channel graph


describe the IK-ACh current
Muscarinic M2, via β/γ subunit of G protein direclty coupled to K+ channel
acti vates the KGIRK1 channel
Leads to hyperpolarisation of cardiac cells = fewer APs
thast how Psympathetics slow HR
describe the IK-ATP current
- These channels (KATP Channels) open in the presence of low intracellular ATP, but close as intracellular ATP rises
- In the pancreatic ß cell their closure leads to insulin release
- In the heart they seem to be protective under conditions of hypoxia
what drug opens KATP Cahnnels - whats it used to treat>
influenced by sulphonylurea drugs to stimulate insulin secretion in Type 2 (non-insulin-dependent) diabetes mellitus.
also targeted by some antihypertesive drugs
describe how beta islets cells work - with glucose dependant insulin release
Glucose is metabolised
→ ATP is produced
→ ATP closes ATP-sensitive Kir channels
→ Cell depolarises
→ Voltage-activated Ca2+ channels open
→ Insulin is released
→ Ca2+-activated K+ channels open
→ Cell repolarises
which APs are from which part of the ehart


In the sinoatrial and atrioventricular nodes the membrane potential only reaches -__ mV (compared with -__ to -__ mV in the ventricles)
In the sinoatrial and atrioventricular nodes the membrane potential only reaches -60 mV (compared with -80 to -90 mV in the ventricles)
tough label


label the currents








Mutations in Voltage-gated K+ channels
what happens when the circles mutate?
what hapens when the squares and triangles mutate
A mutation in the loop connecting domains III and IV of the cardiac voltage-gated Na+ channel produces?

- Circles: episodic ataxia, characterised by attacks of imbalance, uncoordinated motor function, nausea and vertigo
- Squares and triangles: LQT1 and LQT2 - two types of ‘Long QT syndrome’
- loop connecting 3 and 4: LQT3

Long QT syndrome is one of the causes of …..
Long QT syndrome is one of the causes of ‘Sudden Adult Death Syndrome’ (‘SADS’ also known as ‘Sudden Arrhythmic Death Syndrome).
SADS can occur in individuals suffering from a range of different conditions. These include ……
SADS can occur in individuals suffering from a range of different conditions. These include hypertrophic cardiomyopathy and dilated cardiomyopathy as well as Long QT syndrome, where the heart may seem otherwise normal in routine tests.
The QT interval can also be extended by a number of medicines, ……
The QT interval can also be extended by a number of medicines, notably some antibiotics and antipsychotics, with the same poor outcomes.
In most cases of LQT syndrome: what drug? can be used prophylactically to prevent attacks
In most cases ß1-adrenergic antagonists can be used prophylactically to prevent attacks
describe how teh pacemaker current produces APs?
- Nodal tissue - spontaneous APs are gnerated
- Nodal tissue doesnt have stable resting potential
- Potential gradually drifts towards the gating potential for Ca2+ channels
- Special current

Parasympathetic and sympathetic nerves can ______ the production of action potentials, but the intrinsic rhythm of the heart is provided by the spontaneous action potentials generated by the nodal tissue, which are transmitted throughout the myocardium by the conductive system
Parasympathetic and sympathetic nerves can modulate the production of action potentials, but the intrinsic rhythm of the heart is provided by the spontaneous action potentials generated by the nodal tissue, which are transmitted throughout the myocardium by the conductive system
describe the funny current
- HCN (hyperpolarization-activated and cyclic nucleotide-gated) channels open on hyperpolarisation and close on depolarisation (like inward- rectifying K+ channels)
- They are almost as permeable to Na+ as they are to K+
- On hyperpolarisation of the cell, Na+ begins to enter and this leads to a slow depolarisation and ultimately an action potential
- Sympathetic stimulation increases the rate of firing
how are HCN channels modulated by the sympathetic nervous system
n important feature of these channels is that they are activated directly by cAMP, rather than through cAMP-mediated PKA phosphorylation
…………….channels generate the peak of the nodal action potential and voltage-gated K+ channels result in repolarisation.

L and T-type Ca2+ channels generate the peak of the nodal action potential and voltage-gated K+ channels result in repolarisation.
where does cAMP bind to hte HCN channels?
the C terminus binds cAMP as well as cyclic neucleotides
(since theyre cyclic nuceotide gated)
HCN_, which is present not only in the heart, but also in the brain.
HCN_ and HCN_ are the types of HCN channel subunits that are most abundantly expressed in the heart, the former throughout the heart and the latter in the pacemaker regions and in Purkinje fibres
HCN1, which is present not only in the heart, but also in the brain. HCN2 and HCN4 are the types of HCN channel subunits that are most abundantly expressed in the heart, the former throughout the heart and the latter in the pacemaker regions and in Purkinje fibres
Four HCN subunit types have been identified in vertebrates (HCN1-4). Other than in the heart they mostly appear in the ____
Four HCN subunit types have been identified in vertebrates (HCN1-4). Other than in the heart they mostly appear in the brain
Sympathetic stimulation _____ the rate of contraction of the heart and parasympathetic stimulation ______ it.
Sympathetic stimulation increases the rate of contraction of the heart and parasympathetic stimulation slows it.
t or F
Since the sinoatrial node provides the impulse for action potential propagation, any change in the heart rate must be mediated by the potentials produced by the sinoatrial node.
T
label the Modulation of spontaneous action potentials in an isolated sinoatrial cell from a rabbit heart.


Catecholamines effect on teh heat?
Catecholamines accelerate the heart and increase the strength of contraction (a positive chronotropic and inotropic effect)
acetylecholine effect on the heart
Acetylcholine (negative chronotropic and inotropic effect)
fat
mamba


