Biochemistry - Comp Exam Flashcards
Compare activation and inactivation as methods of regulation.
Activation vs. Inactivation

Compare allosteric and competitive effectors in enzyme regulation.
Allosteric vs. Competitive Effectors

Compare covalent and noncovalent bonds.
Covalent Bonds vs. Noncovalent Bonds
- Covalent bonds:
- Strong
- Short
- Share electrons
- Noncovalent bonds:
- Weak
- Long
- Attraction only

Compare glycogen phosphorylase in liver and muscle.
Glycogen Phosphorylase - Liver vs. Muscle
- Liver and muscle forms of GP are isozymes, products of separate genes.
- Differ in their sensitivities to regulatory molecules.
- Liver enzyme:
- Inactivated by free glucose (indicator of blood sugar levels)
- Unaffected by AMP.
- Mutation in liver GP causes Hers disease.
- Muscle enzyme:
- Allosterically activated by AMP (measure of low energy status of cell).
- Mutation in muscle GP causes McArdle syndrome.
Compare glycolysis and gluconeogenesis.
Glycolysis vs Gluconeogenesis
- Glycolysis generates ATP, gluconeogenesis consumes ATP.
- Energy charge (ATP/ADP ratio) determines which pathway will be most active.
- Gluconeogenesis and glycolysis are reciprocally regulated.

Compare reversible and irreversible covalent modification in enzyme regulation.
Reversible vs. Irreversible Covalent Modification

Compare the 3 irreversible steps of glycolysis to the equivalent steps in gluconeogenesis.
Gluconeogenesis vs Glycolysis

Compare the kinetics of transporter-mediated diffusion to simple diffusion and channel-mediated transport.
Passive Transport Kinetics
- Straight line = zero-order.
- Curved line = first-order.

Describe a Lineweaver-Burk plot.
Lineweaver-Burk Plot
- Called a double reciprocal plot.
- Allows us to convert rates to a linear graph.

Describe ABC transporters.
ATP-Binding Cassette Transporters
- 150 ABC transporter genes in human genome.
- Mechanism:
- The catalytic cycle begins with the transporter free of both ATP and substrate. While the distance between the ATP-binding cassettes in this form may vary with the individual transporter, the substrate binding region of the transporter faces inward.
- Substrate enters the central cavity of the transporter from inside the cell. Substrate binding induces conformational changes in the ATP-binding cassettes that increase their affinity for ATP.
- ATP binds to the ATP-binding cassettes, changing their conformations so that the two domains interact strongly with one another. The close interaction of the ABCs reorients the transmembrane helices such that the substrate binding site is now facing outside the cell.
- The outward facing conformation of the transporter has reduced affinity for the substrate, enabling the release of the substrate on the opposite face of the membrane.
- The hydrolysis of ATP and the release of ADP and inorganic phosphate reset the transporter for another cycle.
- Example - multi-drug resistance.

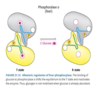
Describe allosteric regulation of glycogen phosphorylase (GP) in the liver.
Glycogen Phosphorylase - Allosteric Regulation in Liver
- Default “a” form or active form.
-
Inactivated by glucose:
- Glucose binds to active site and stabilizes conformation in the inactive T state.
- When glucose levels high, no need for glycogen breakdown (which will make more glucose).
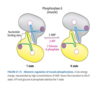
Describe allosteric regulation of glycogen phosphorylase (GP) in the muscle.
Glycogen Phosphorylase - Allosteric Regulation in Muscle
- Default “b” form or inactive form.
-
Activated by AMP.
- Binds to active site and stabilizes conformation of b in the active R state.
- During muscle contraction ATP converted to AMP by myosin and adenylate kinase signaling the GP to breakdown glycogen.
-
ATP and Gluc-6-phosphate are negative allosteric regulators.
- Under normal physiological conditions GP inactive because of inhibitory effect of ATP and Gluc-6-phosphate.
Describe allostery.
Allostery
- Binding that does not occur at the active site.
- Heteroallostery - modulator and substrate are different.
- Homoallostery - modulator and substrate are the same.
Describe aspartate transcarbamoylase (ATCase).
De Novo Pyrimidine Synthesis - Aspartate Transcarbamoylase (ATCase)
- Catalyzes reaction between carbamoyl phosphate and aspartate to form carbamoylaspartate.
- 2 allosteric regulators:
- ATP activates.
- CTP inhibits.

Describe base pairing in nucleic acids.
Nucleic Acids - Base Pairing
- Purines always base-pair with pyrimidines, which creates an antiparallel double helix.
Describe biochemical strategies to drive an unfavorable reaction.
Biochemical Strategies for Driving Unfavorable Reactions
- Maintain Q < K
- Example: create a pathway to use up the products.
- Couple reaction to a highly favorable reaction.
- Example: ATP hydrolysis.
- ΔG values can be summed.
Describe branch transfer and release of glucose in glycogenolysis.
Glycogenolysis - Branch Transfer and Release of Glucose
- Transferase transfers a block of 3 of the remaining 4 glucose to the non-reducing end of the main chain forming an α-1,4 bond.
- Debranching enzyme or α-1,6 glucosidase cleaves the α-1,6 bond of the single remaining glucose residue to release the free glucose. Glucose phosphorylated by hexokinase.
- Transferase and α-1,6 glucosidase convert branched glycogen into a linear structure for further action by GP.

Describe carbamoyl phosphate synthetase II (CPSII).
Carbamoyl Phosphate Synthetase II (CPSII)
- First step in pyrimidine synthesis.
- Has a channel, through which the intermediates travel.
- Active sites are called ATP-grasp folds, which surrounds and holds ATP in position for a nucleophilic attack at the γ phosphoryl group.
- Net of 3 reactions.
- Requires 2 ATP.
- Uses glutamine as amine donor.

Describe catabolism of nucleotides.
Catabolism of Nucleotides
- Purines:
- Final product of catabolism is Uric acid.
- Pyrimidines:
- Final product of catabolism is β-Ureidopropionic acid.
- β-Ureidopropionic acid can be converted to alanine + CO2 + NH3 by the enzyme Ureidopropionase to complete pyrimidine catabolism.
- Final product of catabolism is β-Ureidopropionic acid.
Describe competitive inhibition.
Inhibition - Competitive
- Competing with S for active site.
- Lineweaver-Burk plots for rate with and without inhibitor, cross at y-intercept.
- νmax is constant.
- Km is variable.

Describe complex I of the ETC.
ETC - Complex I
- Complex I aka NADH dehydrogenase aka NADH-Q oxidoreductase.
- Large protein (>900 kDa, with 46 polypeptide chains).
- Encoded by both nuclear and mitochondrial genes.
- First point of entry of electrons from NADH.
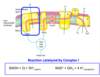
Describe complex II of the ETC.
ETC - Complex II
- FADH2 enters the ETC through Succinate-Q reductase complex (Complex II).
- This complex connects TCA to Oxphos (Succinate Dehydrogenase).
- FADH2 does not leave the complex.
- Its electrons transferred to FeS and then to Q to form QH2.
- Does not pump protons.
- Consequently less ATP synthesized from oxidation of FADH2.
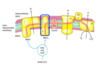
Describe complex III of the ETC.
ETC - Complex III
- Electrons from QH2 are passed on to cytochrome c by cytochrome c oxidoreductase aka Complex III.
- Flow of electrons through this complex leads to transport of 2 protons to cytoplasmic side.
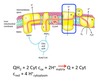
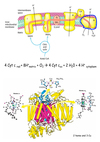
Describe complex IV of the ETC.
ETC - Complex IV
- Last complex aka Complex IV.
- Cyt c oxidase catalyzes transfer of electrons from reduced Cyt c to molecular oxygen, the final acceptor.
- Makes these reactions aerobic.
- Makes humans “breathe”.
- Four electrons funneled to oxygen to reduce it to water.
- Concomitantly protons are pumped from matrix to cytoplasmic side of inner membrane.
Describe configurational isomers.
Isomers - Configurational
- Have chiral Cs.
- 2 types:
- Diastereomers - isomers, with multiple chiral centers, that are not mirror images of each other.
- Enantiomers - isomers, with a single asymmetrical C, that are mirror images of each other.
- Example:
- D -glyceraldehyde and L -glyceraldehyde.
- Example:

Describe conformational isomers.
Isomers - Conformational
- Reversible rotational changes.
- Pyranose rings (6C):
- 2 types:
- Chair
- Substituents on the ring carbon atoms have two orientations:
- Axial - bonds are nearly perpendicular to the average plane of the ring.
- Substituents can sterically hinder each other if they emerge on the same side of the ring
- Equatorial - bonds are nearly parallel to this plane.
- Substituents are less crowded.
- Axial - bonds are nearly perpendicular to the average plane of the ring.
- Substituents on the ring carbon atoms have two orientations:
- Boat
- Chair
- Chair form of β-D-glucopyranose predominates because all axial positions are occupied by hydrogen atoms. The bulkier -OH and -CH2OH groups emerge at the less-hindered periphery.
- Boat form of glucose is disfavored because it is quite sterically hindered.
- 2 types:
- Furanose rings (5C):
- Pucker - 4 atoms nearly coplanar with 1 atom ~0.5 A out of plane.
- 2 conformations:
- C-2-endo - C2 is out of plane on the same side as C5.
- C-3-endo - C3 is out of plane on the same side as C5.
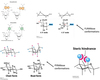
Describe constitutional isomers.
Isomers - Constitutional
- Order of atoms changes.
- Called tautomers:

Describe conversion from Fisher to Haworth projections.
Converting Fisher to Haworth
- Determine 5 or 6 C ring.
- Carbonyl C goes 1 position CW from O.
- Number Cs CW.
- The =O becomes OH:
- α - OH down.
- β - OH up.
- L side of Fisher goes up, R side goes down.
- Until you reach last C before O in the ring.
- Remaining Cs are rotationally ambiguous.

Describe cooperativity.
Enzymes - Cooperativity
- Binding of each subsequent ligand influences the affinity (strength of interaction) of the next ligand to bind an active site.
Describe cooperativity.
Cooperativity
- Binding of an O2 molecule to heme facilitates the binding of O2 to another heme.
- Conformational change in one globin subunit induces a conformational change in another subunit.
- Causing increase in affinity with each successive O2 molecule that binds.
- Also applies to release of O2 in tissues.
- Each successive loss of an O2 molecule decreases affinity.
- Cooperativity is what makes Hb a good O2 carrier.
- 2 models:
- Sequential model:
- Each subunit of hemoglobin exists in T or R state.
- Each subunit that binds O2, causes the adjacent subunit to change from T to R sequentially.
- Concerted model:
- All subunits exist in T or R state.
- Strongly favors T state when no O2 bound.
- Strongly favors R state when O2 is bound.
- Sequential model:

Describe cysteine’s ability to form a disulfide bond.
Cysteine
- Only AA capable of making disulfide bond through a redox reaction.
- All proteins with disulfide bonds must have at least 2 cysteines.

Describe de novo synthesis of purines.
De Novo Synthesis of Purines
- Starts with PRPP and forms the nitrogenous base on the ribose ring, as opposed to pyrimidine synthesis where the nitrogenous base is formed first and then added to PRPP.
- Branched pathway, from inosine monophosphate (IMP) we can either make GMP or AMP.
- Cytoplasmic
- Precursors:
- NH3 from Gln.
- Gly, Asp
- N10-formyl-THF (Vitamin B9)
- HCO3-
- Regulation:
- Feedback by purines.
- Reactions:
- 1-3 - form the smaller, 5-member ring:
- Swaps PPi on PRPP for NH3 from Gln.
- Adds glycine.
- Adds a formyl group from N10-formyl THF to complete the ring.
- 4-10 form the 6-member ring:
- Adds NH3 from Gln to start the second ring.
- Closes the 5-member ring.
- Adds CO2 from HCO3- first to Gln (N) then to Gly (α-C).
- Adds Asp at the carboxyl.
- Releases Fumarate.
- Second addition of a formyl group from (another) N10-formyl THF (vitamin B9), completing the 6-membered ring.
- Closes the 6-membered ring, forming hypoxanthine.
- 1-3 - form the smaller, 5-member ring:
- Double ring structure is hypoxanthine, entire nucleotide is called inosinate (IMP).
- Reactions 1-10 occur in one enzyme complex consisting of 6 enzymes.
- 1-4 occur in individual proteins.
- 2,3,5; 6,7; 9,10 carried out on 3 mutlifunctional proteins.
- Carried out in close proximity so intermediates can easily be passed from domain to domain.
- 2 additional enzymes provide the N10-formyl-THF.
- IMP can then make either AMP or GMP.
- AMP and GMP get converted through two kinases to ATP and GTP.

Describe de novo synthesis of pyrimidines.
Pyrimidines - De Novo Synthesis
- Unidirectional pathway.
- Cytoplasmic.
- Precursors:
- -NH3 from Gln.
- Asp
- HCO3-
- Allosteric Regulation:
- Pyr inhibits (C)
- Pur activates (A/G)
- Ring is synthesized first then attached to a ribose phosphate to form a pyrimidine nucleotide.
- Assembled from bicarb, aspartate, and NH3.
- NH3 may already be present in solution but usually obtained from glutamine.
- Reactions:
- 6 reactions to form pyrimidine:
- CAD - multifunctional, eukaryotic protein performs the functions of:
- Carbamoyl phosphate synthetase II (CPSII).
- Aspartate transcarbamoylase (ATCase).
- 2 allosteric regulators:
- ATP activates.
- CTP inhibits.
- 2 allosteric regulators:
- Dihydroorotase
- Dihydroorotase dehydrogenase.
- Located in mitochondria.
- UMP synthetase:
- Multifunctional protein, performs two functions.
- Nucleoside monophosphate kinases.
- Specific to each NMP
- Nucleoside Diphosphate Kinase.
- Broad specificity.
- CTP synthetase
- 2 allosteric regulators just like ATCase:
- CTP inhibits.
- GTP activates.
- 2 allosteric regulators just like ATCase:
- CAD - multifunctional, eukaryotic protein performs the functions of:
- 6 reactions to form pyrimidine:
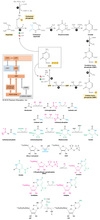
Describe diastereomers.
Isomers - Diastereomers
- 2 types:
- Epimers - diastereoisomers differing in configuration at only a single asymmetric center.
- Note that D-glucose and D-mannose differ in configuration only at C-2, the carbon atom in the second position. Thus, D-glucose and D-mannose are epimeric at C-2; D-glucose and D-galactose are epimeric at C-4.
- Anomers - when carbohydrates form a cyclic hemiacetal (ring), creating an additional asymmetric center.
- In glucose, C-1 (the carbonyl carbon atom in the open-chain form) becomes an asymmetric center (anomeric C). Thus, two ring structures can be formed:
- α-D-glucopyranose and β-D-glucopyranose.
- α designation means that the hydroxyl group attached to C-1 is on the opposite side of the ring as C-6.
- β designation means that the hydroxyl group is on the same side of the ring as C-6.
- In glucose, C-1 (the carbonyl carbon atom in the open-chain form) becomes an asymmetric center (anomeric C). Thus, two ring structures can be formed:
- Epimers - diastereoisomers differing in configuration at only a single asymmetric center.
- Monosaccharides made up of more than three carbon atoms have multiple asymmetric carbons, and so they can exist not only as enantiomers but also as diastereoisomers.
- The number of possible stereoisomers equals 2n, where n is the number of asymmetric carbon atoms.
- Six-carbon aldose with 4 asymmetric carbon atoms can exist as 16 possible diastereoisomers, of which glucose is one such isomer.

Describe dihydroorotase.
De Novo Pyrimidine Synthesis - Dihydooritase
- Assists carbamoylaspartate to cyclize forming dihydroorotate.
- Dihydroorotate is oxidized by NAD+ to form orotate.
- Opposite of hydrolase.
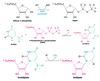
Describe dihydroorotate dehydrogenase.
De Novo Pyrimidine Synthesis - Dihydroorotate Dehydrogenase
- Located in inner membrane of mitochondria.
- Converts dyhydroorotate to orotate by removing water and forming double bond.
- Uses FMN to accept e- forming FMNH2.
- FMNH2 passes e- to coenzyme Q in ETC forming QH2.
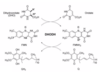
Describe dipole electrostatic interactions.
Electrostatic Interactions - Dipoles
- Dipole - means having a partial charge.
- Permanent (polar)
- Induced (polarizable)

Describe disaccharides.
Disaccharides
- Consists of two sugars joined by an O-glycosidic bond.
- Name gives all info.
- α - hydroxyl points down.
- β - hydroxyl points up.
- Three common disaccharides:
- Sucrose (common table sugar):
- Obtained commercially from sugar cane or sugar beets.
- Anomeric carbon atoms of a glucose unit and a fructose unit are joined in this disaccharide.
- Configuration is α for glucose and β for fructose.
- Can be cleaved into its component monosaccharides by the enzyme sucrase.
- Lactose:
- Consists of galactose joined to glucose by a β-1,4-glycosidic linkage.
- Hydrolyzed to these monosaccharides by lactase in human beings and by β-galactosidase in bacteria.
- Maltose
- Consists of two glucose units joined by an α-1,4-glycosidic linkage.
- Comes from the hydrolysis of large polymeric oligosaccharides such as starch and glycogen.
- Hydrolyzed to glucose by maltase.
- Sucrose (common table sugar):
- Sucrase, lactase, and maltase are located on the outer surfaces of epithelial cells lining the small intestine.
- The cleavage products of sucrose, lactose, and maltose can be further processed to provide energy in the form of ATP.

Describe dispersion (van der Waals forces) electrostatic interactions.
Electrostatic Interactions - Dispersion

Describe enzyme inhibitors.
Enzymes - Inhibitors
- Can be:
- Irreversible
- Reversible
- Reversible:
- Use noncovalent interactions to bind.
- 3 types:
- Competitive
- Noncompetitive (allosteric)
- Uncompetitive (allosteric)
Describe equilibrium.
Equilibrium
- Dynamic equilibrium - making and/or breaking chemical bonds.
- Most reactions are reversible.
- Does not mean equal concentrations.

Describe essential vs non-essential amino acids.
Amino Acids - Essential vs Non-Essential
- Essential - must consume, cannot be synthesized by the body.
- 2 exceptions - argenine and methionine can be synthesized, but are considered essential because consuption > production.
- Non-essential - body can synthesize.
- 1 exception - tyrosine can be made through secondary synthesis by adding an OH to phenylalanine, however it is only non-essential as long as there is an adequate supply of phenylalanine.

Describe FA translocation to mt matrix.
Fatty Acids - Translocation to mt Matrix

Describe facilitated diffusion.
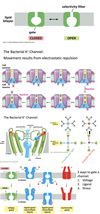
Facilitated Diffusion - Ion Channels
- 2 important features:
- Selectivity filter:
- Varies based on ion allowed to pass.
- Example K+:
- Although Na+ is smaller than K+, K+ channels are 100x more permeable to K+.
- Due to O atoms in selectivity filter.
- Make the dehydration of K+ more favorable than the dehydration of Na+.
- Gate (three ways):
- Voltage
- Ligand
- Stress
- Selectivity filter:
- Bacterial K+ channel:
- Movement results from electrostatic repulsion.
Describe fatty acid synthase (FAS).
Fatty Acid Synthase (FAS)
- Large multi-enzyme complex.
- Composed of 2 identical dimers (260 kDa each).
- Two dimers arranged in head to tail conformation.
- Each has 7 enzyme activities and an acyl carrier protein (ACP).

Describe fucose.
Fucose
- Fucose is wierd.
- Usually when we replace the -OH we are left with an O or N except in the case of fucose.
- Galactose derivative
- Know if there is a methyl attached to C5 that it is fucose.
- Only L-monosaccharide made and used by mammals.
- Part of A/B/O blood antigens.
- Excess free fucose in blood = liver damage, cancer, diabetes, heart disease.

Describe G6PD regulation.
Glucose 6-Phosphate Dehydrogenase - Regulation
- Regulation by:
- Transcription/Translation control.
- Location in cell.
- Post-translational controls.
- Activators:
- Dimerization
- Transcription factors for antioxidant genes.
- Cell cycle and synthesis activators.
- Insulin
- Inhibitors:
- Phosphorylation
- Apoptosis-signaling proteins.

Describe G6PD.
Glucose 6-Phosphate Dehydrogenase
- First step in the oxidative phase of PPP.
- Catalyzes the dehydrogenation of G6P to 6-phosphoglucono-δ-lactone.
- Essentially irreversible.
- Rate limiting.
- 3 forms:
- Monomer
- Dimer
- Tetramer.
- NADPH functions as both a substrate and a coenzyme.

Describe Gibbs free energy.
Gibbs Free Energy

Describe gluconeogenesis.
Gluconeogenesis
- Occurs in liver and kidney.
- Synthesis of glucose from non-carbohydrate precursors.
- Pathway not a reversal of glycolysis.
- Pathway converts pyruvate into glucose.
- Major precursors are lactate, amino acids, and glycerol.
- “By-passes” the irreversible steps of glycolysis through four enzymes not present in glycolysis:
- Pyruvate carboxylase
- Phosphoenolpyruvate carboxykinase
- Fructose 1,6-bisphosphatase
- Glucose 6-phosphatase

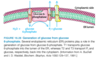
Describe glucose 6-phosphatase.
Glucose 6-Phosphatase
- Enzyme located in the lumen of the endoplasmic reticulum (ER).
- T1 transports Glucose 6-phosphate to ER.
- T2 transports Inorganic phosphate back into cytosol.
- T3 transports Glucose back to cytoplasm.
Describe glucose-6-phosphate dehydrogenase deficiency (G6PDD).
Glucose-6-Phosphate Dehydrogenase Deficiency (G6PDD)
- Prevalence:
- Most common genetic disease.
- ~400 million people have worldwide.
- ~7.5% allele frequency.
- Genotype/Mutation(s):
- X-linked, recessive.
- ~200 reported missense mutations.
- Phenotypic manifestations:

Describe glycogenesis.
Glycogenesis
- 3 Steps:
- Trapping and Activation of Glucose:
- Glucokinase/hexokinase in cytosol of hepatocytes and muscle cells catalyze phosphorylation of glucose to glucose-6-phosphate.
- Phosphoglucomutase then reversibly isomerizes glucose-6-phosphate to glucose-1-phosphate.
- Uridine diphosphate(UDP)-glucose pyrophosphorylase then transfers the glucose-1-phosphate to uridine triphosphate (UTP) which generates UDP-glucose (active form of glucose).
- Breakdown of pyrophosphate to Pi generates energy.
- Elongation of a glycogen primer:
- Preexisting glycogen
polymer serves as primer to
which glucose units are
added. - Glycogen synthase (rate
limiting enzyme). Catalyzes
transfer of glucose from
UDP-glucose to non-
reducing end of glycogen
chain. Forms α-1,4
glycosidic bonds between
glucose molecules.
- Preexisting glycogen
- Branching of glycogen chains:
- When glycogen chain reaches 11 residues, a fragment of the chain (about 7 residues long) is broken off at an α -1, 4 link and reattached elsewhere through an α
-1,6 link by glucosyl (4:6) transferase. - The new branch point must be at least 4 residues away from a preexisting branch.
- Branching increases solubility of glycogen and increases number of terminal non-reducing ends.
- Increases rate at which glycogen can be synthesized and degraded.
- When glycogen chain reaches 11 residues, a fragment of the chain (about 7 residues long) is broken off at an α -1, 4 link and reattached elsewhere through an α
- Trapping and Activation of Glucose:

Describe glycogenolysis.
Glycogenolysis
- Glycogen broken down to release glucose-1-phosphate.
- Glycogen remnant remodeled to permit further degradation.
- Glucose-1-phosphate converted to glucose-6-phosphate.
- Glycolysis
- Free glucose for release into blood stream.
- Pentose phosphate pathway – NADPH and ribose derivative.
- Four key enzymes:
- One to degrade glycogen (chain shortening).
- Two to remodel glycogen remnants.
- One to convert glycogen breakdown product suitable for further metabolism.

Describe glycolysis.
Glycolysis
- Involves a sequence of reactions that metabolizes:
- 1 molecule of glucose to 2 molecules of pyruvate and generates 2 ATP.
- Anaerobic process.
- Used to provide energy when requirements outpace O2 delivery.
- Pyruvate completely oxidized under aerobic conditions, generates much more ATP.
Describe gout.
Gout
- At high concentrations, urate crystallizes.
- Chronic elevated levels of urate in the blood (hyperuricemia) results in uric acid crystals collecting in joints = GOUT.
- Inflammation
- Arthritis
- Joint degeneration
- Many pathologies can produce gout symptoms, one is HPRT deficiency (Lesch-Nyhan Syndrome).
Describe how isozymes regulate.
Enzyme Regulation - Isozymes
- Can “mix and match” subunits.
- Catalyze same reaction but with different efficiencies.
- Tissue specificity - compartmentalized isozymes, different isozymes present in different tissues.
- Development - temporal expression of isozymes, different isozymes present at different time frames in development (e.g. HbF -> HbA).
Describe hydrolases.
Enzymes - Hydrolases
- Break a chemical bond by adding water across it (hydrolysis)

Describe integral membrane proteins.
Integral Membrane Proteins
- Interact extensively with the hydrocarbon chains of membrane lipids, and can be released only by agents that compete for these nonpolar interactions.
- Transmembrane regions of proteins have hydrophobic AAs displayed on their surfaces.
- α-helices are most common structure for transmembrane region, but can be beta sheet as in bacterial porin.
- Cotranslational insertion - feed into translocation channel directly from ribosome.
Describe irreversible inhibitors.
Inhibition - Irreversible
- Inhibition is permanent.
- Form covalent interactions.

Describe isomerases.
Enzymes - Isomerases
- Rearrange order of atoms in a molecule (isomerization).

Compare isozymes and enzyme level control in enzyme regulation.
Isozymes vs Enzyme Level Control

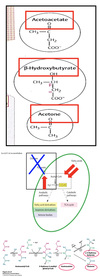
Describe ketone bodies.
Ketone Bodies
- Water-Soluble and Acidic Compounds:
- Acetoacetate
- β-Hydroxybutyrate
- Acetone
- Produced in Liver Only:
- Provide energy for peripheral tissues during fasting & brain during starvation!!!!
- Fasting/Starvation = Excessive β-oxidation of FAs –> ↑[Acetyl-CoA].
Describe ligases.
Enzymes - Ligases
- Paste two pieces together (make a chemical bond), uses ATP.
- Activated carriers:
- +/- aldehyde group (-COH):
- TPP
- +/- acyl group (-COR):
- CoASH
- Lipoamide
- +/- CO2 group:
- Biotin
- +/- aldehyde group (-COH):

Describe lyases.
Enzymes - Lyases
- Break chemical bond without using water.

Describe lysosomal glycogenolysis.
Glycogenolysis - Lysosomal
- Small amount of glycogenolysis occurs in lysosomes.
- In Pompe disease lysosomes become engorged with glycogen because they lack α-1,6-glucosidase, a hydrolytic enzyme confined to these organelles.
Describe malonyl-CoA.
Malonyl CoA
- Substrate for Fatty Acid Synthase (FAS).
- Regulator – inhibits carnitine acyltransferase (rate limiting step in FA degradation).
- Prevents FA synthesis and degradation from occurring simultaneously.
Describe membrane proteins.
Membrane Proteins
- Constitute ~30% of the proteome.
- Receive external signals.
- Transmit signals into cytoplasm.
- Transmit signals to another cell.
- Allow solutes through the membrane.
- Help to determine membrane thickness and rigidity.
Describe Mode 1 of the PPP.
Pentose Phosphate Pathway - Mode 1
- Utilized when the needs of ribose 5-phosphate >> NADPH.
- Produces primarily ribose 5-phosphate.
- Example:
- Rapidly dividing cells that need ribose 5-phosphate for synthesis of DNA precursors.

Describe Mode 2 of the PPP.
Pentose Phosphate Pathway - Mode 2
- Utilized when the needs for NADPH and ribose 5-phosphate are equal.
- Only proceeds through the oxidative phase.

Describe Mode 3 of the PPP.
Pentose Phosphate Pathway - Mode 3
- Utilized when the need for NADPH >> ribose 5-phosphate.
- Produces primarily NADPH.
- Example:
- Cells undergoing FA biosynthesis (adipose).

Describe Mode 4 in the PPP.
Pentose Phosphate Pathway - Mode 4
- Utilized when both NADPH and ATP are needed.
- Produces NADPH and ATP.

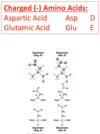
Describe negatively charged amino acids.
Amino Acids - Negatively Charged
- Can accept a proton.
Describe noncompetitive inhibition.
Inhibition - Noncompetitive
- Inhibitor binds at allosteric site.
- Can bind before or after S binds to active site.
- Lineweaver-Burk plots for rate with and without inhibitor, cross at x-intercept.
- νmax is variable.
- Km is constant.

Describe nucleic acid growth.
Nucleic Acids - Growth
- Biologically, always grows in the 5’ -> 3’ direction.
- In vitro oligonucleotide synthesis proceeds 3’ -> 5’.

Describe oxidative phosphorylation.
Oxidative Phosphorylation
- TCA cycle generates NADH and FADH2.
- In OxPhos these high energy electrons flow through 4 protein complexes called the electron transfer chain (ETC).
- Electrons reduce molecular O2 to water.
- Three of the complexes pump protons from matrix to intermembrane space.
- Protons return to matrix by flowing through another complex called ATP synthase.
- ATP synthesis.
- A successful OxPhos must accomplish the following key goals:
- Transfer electrons from NADH and FADH2 –> O2.
- Establish a proton gradient across the inner mitochondrial membrane H+.
- Synthesize ATP.

Describe oxidoreductases.
Enzymes - Oxidoreductases
- Move electrons (redox reactions)
- Activated carriers: NADH, NADPH, FADH2, FMNH2.

Describe P-type ATPases.
P-Type ATPases
- Use ATP.
- Couple phosphorylation and conformational changes to pump ions across membranes.
- 2 principal conformational states:
- Binding sites open to one side of membrane and binding sites open to other side of membrane.
- Interconversion between conformational states must be coupled with ATP hydrolysis.
- Example - SERCA:
- Pumps Ca2+ from cytosol into SR in skeletal muscle.
- Can bind 2 Ca2+, each coordinated with 7 O atoms.
- 3 domains:
- A domain - serves as actuator, linking changes in N and P domains to the transmembrane part of the enzyme.
- P domain - accepts phosphoryl group.
- N domain - binds the ATP nucleotide
- Catalytic cycle:
- With the release of phosphate, the interactions stabilizing the E2 conformation are lost, and the enzyme everts to the E1 conformation.
- The phosphorylaspartate residue is hydrolyzed to release inorganic phosphate.
- Upon ADP release, the enzyme again changes its overall conformation, including the membrane domain this time. This new conformation is referred to as E2 or E2-P in its phosphorylated form. The process of interconverting the E1 and E2 conformations is sometimes referred to as eversion.
- The catalytic cycle begins with the enzyme in its unphosphorylated state with two calcium ions bound. We will refer to the overall enzyme conformation in this state as E1 with Ca2+ bound, it is E1 -(Ca2+)2. In this conformation, SERCA can bind calcium ions only on the cytoplasmic side of the membrane.
- In the E1 conformation, the enzyme can bind ATP. The N, P, and A domains undergo substantial rearrangement as they close around the bound ATP, but there is no substantial conformational change in the transmembrane domain. The calcium ions are now trapped inside the enzyme.
- The phosphoryl group is then transferred from ATP to Asp 351.
Describe peripheral membrane proteins.
Peripheral Membrane Proteins
- Bound to membrane primarily by electrostatic and H-bond interactions with the head groups of lipids.
- May be bound to the surfaces of integral proteins.
- Some are anchored to the lipid bilayer by a covalently attached hydrophobic chain, such as a fatty acid.
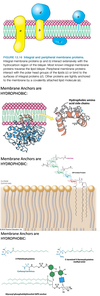
Describe phase 1 of FA synthesis.
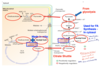
Fatty Acid Synthesis - Phase 1
- Transport of Acetyl CoA from mitochondria to cytoplasm.
- Step 1 - Condensation of Acetyl CoA with oxaloacetate (OAA) to form citrate. Catalyzed by citrate synthase.
- Step 2 - Transport of citrate from mitochondria to cytosol. Via a citrate transporter.
- Step 3 - Citrate converted back to Acetyl CoA and OAA. Catalyzed by citrate lyase** **(check out its regulation in the schematic!!!).
- Acetyl CoA used for FA synthesis in cytoplasm.
- Step 4 - OAA reduced to malate by malate dehydrogenase.
- Regeneration of OAA - Two mechanisms:
- Step 5 - Malate transported into mitochondria via malate-α-ketoglutarate transporter and oxidized to OAA by malate dehydrogenase.
- Step 6 - Cytosolic malate converted to pyruvate by malic enzyme. Pyruvate transported to mitochondria via pyruvate transporter and carboxylated to OAA by pyruvate carboxylase.
Describe phase I of fatty acid breakdown.
Fatty Acid Breakdown - Activation

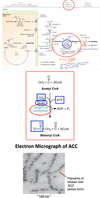
Describe phase II of FA synthesis.
Fatty Acid Synthesis - Phase II
- Acetyl-CoA (2 carbon) is converted to Malonyl CoA (3 carbon) by carboxylation, catalyzed by Acetyl CoA Carboxylase (ACC).
- Rate limiting step of fatty acid biosynthesis.
- ACC adds a CO2 to Acetyl-CoA.
- Uses ATP (for energy) and Biotin as co-factor.
- Exists in dimeric (inactive) or polymeric (active) forms (Check out the regulation of ACC in schematic!!!).
Describe phase II of fatty acid breakdown.
Fatty Acid Breakdown - Phase II (β-Oxidation)
- Acyl CoA dehydrogenase (ACAD) - oxidizes the β carbon to produce FADH2 and trans-enoyl-CoA. FADH2 enters ETC to generate 2 ATP.
- Enoyl CoA hydratase saturates the alkene with water to form β hydroxy acyl CoA.
- β hydroxy acyl CoA dehydrogenase oxidizes the carbon to form ketoacyl CoA and NADH. NADH enters ETC to generate 3 ATP.
- Acyl CoA acyl transferase or ketothiolase attaches Sulfur of CoA to ketone formed from cleavage of acetyl CoA from fatty acyl chain which is shortened by 2C.
- Generate:
- FADH2 (delivers electrons to CoQ/ubiquinone of ETC).
- NADH (delivers electrons to Complex I of ETC).
- Acetyl CoA (enters TCA cycle).
- This group of 4 reactions repeated until FA broken down to acetyl CoA.
Describe phase III of FA synthesis.
Fatty Acid Synthesis - Phase III
- Two carbon units from malonyl CoA are sequentially added to the growing fatty acyl chain in 7 reactions to form palmitate (16:0).
- The reactions of fatty acid synthesis occur on the Fatty Acid Synthase (FAS) Complex.

Describe phosphorylation and dephosphorylation as reversible covalent modifications in regulating enzymes.
Phosphorylation / Dephosphorylation
- Kinases - add phosphates.
- Tyr, Ser, or Thr kinases phosphorylate Tyr, Ser, or Thr residues.
- Phosphatases - remove phosphate.
- Name of kinase indicates on which amino acid the phosphate will be added.
- Why is phophorylation activating?
- Thermodynamics:
- ATP hydrolysis can drive unfavorable reactions (ΔG ≈ -50 kJ/mol).
- Kinetics:
- Physiological processes dictate reaction rate (msec – hrs/rxn).
- Cell processes:
- ATP amounts dictated by metabolism (energy charge)
- Signal transduction amplification (catalytic turnover).
- Shape and Charge Complementarity:
- Each phosphate adds (-2) charge and (3+) H-bonds.
- Thermodynamics:

Describe positively charged amino acids.
Amino Acids - Positively Charged
- Acidic - have a proton available to donate.
- Histidine, although not protonated at physiological pH, has a pKa that is close. Therefore it may be possible to alter the microenvironment protonating histidine.

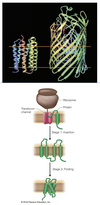
Describe protein structures.
Protein Structure
- Primary - a chain of AA.
- Secondary - local folding of the polypeptide chain.
- Tertiary - folding of the secondary structure.
- Quarternary - interaction of multiple peptides.

Describe PRPP.
PRPP
- 5-Phosphoribosyl-1-pyrophosphate synthetase synthesizes PRPP by adding a pyrophosphate from ATP to ribose-5-phosphate (from the pentose phosphate pathway).
Describe reaction rate and catalysis.
Reaction Rate and Catalysis
- Michaelis-Menten enzymes follow first-order kinetics (rate increases directly proportional to [reactant]).
- Catalysis is not a first-order reaction.
- Product does initially increase with time.
- Eventually reaction reaches point where there is no net change in [S] and [P] (i.e. equilibrium).
- Enzyme is still actively converting S -> P and P-> S.
- Forward rate = reverse rate.
- Kinetics explaned by the formation of an intermediate that can either progress to products or return to reactants.
- There are rate constants for each step and each direction.
- Calculation of reaction rate can be simplified:
- Measure initial rate ν0.
- At t~0 there will either be no P or [P] will be negligible.
- At t~0 whatever P is present was released instantly, so [EP] ~0 (k3>>k-2)
- Changes in concentration will be linear.
- Therefore, [EP] and k-2 can be ignored giving us the Michaelis-Menten equation.
- Slope of linear graph = reaction rate.
- Measure initial rate ν0.

Describe reaction rate.
Reaction Rate
- Number of reactions over time.
- 2 considerations:
- Irreversible
- Reversible
- Need to consider both forward and reverse reactions.
- Equilibrium - when forward rate = reverse rate.
- Exponents indicate # of substrates, equals 1 in first-order equations, often omitted.

Describe regulation of glycolysis.
Glycolysis - Regulation
- Goals:
- To generate ATP during activity:
- ATP levels regulate glycolysis.
- To maintain blood glucose levels:
- To provide building blocks for other pathways (in response to biochemical diversity and need).
- To generate ATP during activity:
- Major regulatory enzymes:
- Hexokinase
- Phosphofructokinase
- Activated by F-2,6-BP
- Inhibited by citrate
- Pyruvate kinase
- Regulated by allosteric effectors and covalent modification.
- Glucokinase
- No hexokinase in liver.
- Glucokinase is not inhibited by glucose 6-phosphate – Glucose permanently trapped.

Describe ribonucleotide reductase.
Ribonucleotide Reductase
- Removes the OH from C2 on NDPs.
- NADPH provides the necessary e- for redox reactions.
- Active site = catalytic site.
- Regulation:
- 2 allosteric sites =
- Activity site.
- “On/off switch.”
- ATP promotes function.
- dATP turns enzyme off by altering subunit contacts.
- Specificity site
- “Volume dial” / how much of and what kind.
- dATP in specificity site:
- Pyrimidines preferred in active site.
- dTTP in specificity site:
- GDP preffered in active site.
- Pyrimidines inhibited.
- dGTP
- ADP preferred in active site.
- Pyrimidines inhibited (only in invertebrates).
- Activity site.
- 2 allosteric sites =

Describe salvage of purines.
Salvage and Catabolism of Purines
- Purines use phosphoribosyltransferases to salvage in one step.
- Adenine phosphoribosyltransferase makes AMP.
- Hypoxanthine-Guanine phosphoribosyltransferase (HGPRT or HPRT) makes IMP/GMP.
- NOTE: Purine metabolism contains more than just A’s and G’s…

Describe secondary active transport.
Secondary Active Transport
- Uses gradient established by primary active transport to power transport.
- All secondary active transporters are symporters.
- Example - Na-glucose cotransport:
- Na+ travels down its concentration gradient.
- Brings glucose with it.
- Example - lactose permease:
- Proton travels down its concentration gradient.
- Drives transport of lactose.

Describe simple diffusion.
Simple Diffusion
- Factors affecting diffusion rates:
- Magnitude of the concentration gradient (larger = faster).
- Size of the molecule (larger = slower).
- Surface area:volume ratio (shape) (higher = faster).
- Temperature (higher = faster).
- Density of the solvent (higher = slower).
- Solubility of solute (nonpolar = soluble).
- Distance to destination (longer = slower).

Describe stage 1 of glycolysis.
Glycolysis - Stage 1
- Trapping of Glucose and Preparation phase:
- No ATP generated.
- Two ATPs consumed.
- Consists of three reactions: phosphorylation, isomerization, a second phosphorylation.
- Strategy of this phase is to trap the glucose in cell and form a compound that can be readily cleaved into 2 phosphorylated three-carbon units.
- 5 steps:
- Glucose phosphorylated to glucose-6-phosphate (G6P). ATP consumed. Enzyme hexokinase (in all tissues) and glucokinase (in liver).
- G6P isomerized to fructose-6-phosphate (F6P). Enzyme phosphoglucoisomerase.
- F6P phosphorylated to fructose-1,6-bisphosphate (F1,6BP). ATP consumed. Enzyme phosphofructokinase (rate limiting enzyme of glycolysis).
- F1,6BP broken down to glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP). Enzyme is aldolase.
- DHAP isomerized to G3P. Enzyme triose phosphate isomerase.

Describe stage 2 of glycolysis.
Glycolysis - Stage 2
- Energy harnessed in GAP (G3P) used to form ATP.
- Steps:
- GAPDH step:
- Oxidative phosphorylation of GAP to form 1,3-BPG.
- Reduce NAD+ to NADH
- 1,3-BPG has high phosphoryl-transfer potential.
- NADH contains a pair of
“high energy” electrons.- Sent to electron transport chain (ETC), play role in oxidative phosphorylation (OXPHOS).
- Phosphoglycerate kinase/mutase steps:
- The kinase converts 1,3 BPG to 3-PG.
- ADP is phosphorylated:
- ATP to form 3-PG.
- Via substrate transfer.
- ATP to form 3-PG.
- The mutase moves phosphate from 3rd to 2nd position (2-PG).
- Remember = 2X
- Enolase/Pyruvate kinase steps:
- Dehydration of 2-PG by enolase forms PEP, an enol with high phosphoryl-transfer potential (unstable).
- Then, Pyruvate kinase transfers phosphoryl group from PEP to ADP to form ATP.
- PEP is converted from unstable enol to pyruvate, a stable ketone.
- This step is irreversible.
- Remember = 2X.
- GAPDH step:

Describe stereoisomers.
Isomers - Stereoisomers
- Have same connectivity but different spatial organizations.
- According to convention, the D and L isomers are determined by the configuration of the asymmetric carbon atom farthest from the aldehyde or keto group.
- Example:
- Glyceraldehyde has a single asymmetric carbon atom and, thus, there are two stereoisomers of this sugar: D -glyceraldehyde and L -glyceraldehyde.
- These molecules are a type of stereoisomer called enantiomers, which are mirror images of each other.

Describe the 4 catalysis strategies.
Catalysis Strategies
- Covalent catalysis - enzyme covalently bonds the transition state (e- transfer).
- Acid-Base catalysis - partial proton transfer to the substrate.
- Approximation:
- Proper spatial orientation and close contact (proximity) of the reactant molecules must occur.
- If both pieces of the puzzle are “captured” and held in the proper orientation, they are more likely to react with one another (entropy reduction).
- Electrostatic catalysis - stabilization of unfavorable charges on the transition state by polarizable side chains in the enzyme and/or metal ions.

Describe the application of ion channels in neuromuscular transmission.
Neuromuscular Transmission - Ion Channel Application
- 5 ion channels work together to contract your muscles:
- Depolarization opens voltage-gated Ca2+ channels.
- Exocytosed acetylcholine opens ligand-gated Na+ channels.
- Local depolarization opens adjacent voltage-gated Na+ channels.
- As the depolarization spreads, voltage gated Ca2+ channels open.
- Coupled Ca2+-release channels embedded in the SR open.

Describe the association and dissociation constants.
Association Constant vs. Dissociation Constant
- Refer to binding (association/dissociation).
- Calculated for reversible reactions at equilibrium.

Describe the breakdown of FAs.
Fatty Acids - Breakdown

Describe the calculation of ATP released from β-oxidation of palmitic acid.
β-Oxidation of Palmitatic Acid - Calculation of ATP
- Palmitate - C16 fatty acid.
- Degradation of palmitoyl CoA requires 7 reaction cycles. In the 7th cycle, the C4 compound thiolyzed to 2 acetyl CoA.
- Equation:
- Palmitoyl CoA + 7FAD + 7NAD+ + 7CoA + 7H2O ——- 7FADH2 + 7 NADH + 7 H+ + 8 Acetyl CoA.
- FADH2 :7X2=14ATP
- NADH : 7 x 3 = 21 ATP
- Acetyl CoA : 8 x 12 = 96 ATP
- Total = 131
- ATP used: 2 (during activation of palmitate):
- Net ATP = 129.
Describe the catalysis model.
Enzymes - Catalysis Model
- Catalysis achieved through:
- Substrate orientation.
- Straining substrate bonds.
- Creating a favorable microenvironment.
- Covalent and/or noncovalent interactions between enzyme and substrate.

Describe the chain shortening process of glycogenolysis.
Glycogenolysis - Chain Shortening
- Glycogen phosphorylase (GP) (rate limiting enzyme) catalyzes the cleavage of glycogen.
- Chain shortening occurs at the non-reducing end of the polymer.
- GP adds an orthophosphate and releases a glucose residue as glucose-1-phosphate.
- Uses pyridoxal phosphate (vitamin B6) as a cofactor.
- Phosphorolysis of glucose residues continues till the GP gets within 4 residues of the α-1,6 linkage of a branch point.

Describe the citric acid cycle.
Citric Acid Cycle
- Condensation of 4-carbon oxaloacetate and 2-carbon acetyl group of acetyl CoA.
- Oxaloacetate reacts with acetyl CoA + water yields citrate and CoA.
- Catalyzed by citrate synthase.
- Citrate isomerizes to isocitrate:
- Hydroxl group of citrate is not in proper location for oxidative decarboxylation.
- Dehydration/hydration moves OH atoms via the enzyme aconitase (uses iron-sulfur cluster to bind citrate).
- Isocitrate -> α-ketoglutarate:
- Isocitrate dehydrogenase.
- First of 4 oxidation-reduction reactions.
- Unstable intermediate, oxalosuccinate, loses CO2 while bound to the enzyme.
- This step is important in determining the rate of the cycle.
- Rate limiting step.
- α-ketoglutarate -> Succinyl-CoA:
- α-ketoglutarate dehydrogenase
- Complex similar to pyruvate dehydrogenase.
- Catalyzes similar reaction.
- Both reactions decarboxylate an α-ketoacid and create a thioester linkage with CoA.
- Succinyl-CoA -> Succinate:
- Succinyl-CoA synthetase
- Succinyl-CoA contains a high energy thioester bond (ΔG0’ similar to ATP).
- Only step that directly yields a high energy phospho-transfer compound (GTP, ATP) (substrate level phosphorylation).
- Two isozymic forms:
- GTP form in tissues that perform many anabolic reactions (liver).
- ATP form in tissues that perform large amounts of cellular respiration (skeletal and heart muscle).
- Succinate -> Fumarate:
- Succinate dehydrogenase catalyzes formation of fumarate while generating FADH2.
- The enzyme is located in the inner mitochondrial membrane directly associated with electron transport chain (Complex-II).
- FADH2 is actually not released from the enzyme, but electrons are passed directly to Co-Q in the electron transport chain.
- Fumarate -> L-Malate:
- Fumarase catalyzes the hydration of fumarate to L-malate.
- Malate -> Oxeloacetate:
- Oxidation of malate has a positive standard free energy.
-
Malate dehydrogenase recognizes specifically L-Malate.
- Reactions are driven by the use of the products:
- Oxaloacetate - citrate synthase.
- NADH - electron transport chain.
- Reactions are driven by the use of the products:

Describe the components of the ETC.
ETC - Components
- Electrons transferred from NADH to O2 via three large protein complexes:
- NADH Q oxidoreductase (Complex I).
- Q cytochrome c oxidoreductase (Complex III).
- Cytochrome c oxidase (Complex IV).
- Electron flow exergonic.
- Powers flow of protons across inner membrane.
- Fourth complex is Succinate Q reductase (Complex II).
- Has succinate dehydrogenase which generates FADH2 in TCA cycle.
- Electrons from FADH2 enter through Q cytochrome c oxidoreductase.
- Succinate Q reductase does not pump protons.

Describe the concept of the folding funnel.
Protein Folding - Folding Funnel
- As entropy decreases, degree of folding increases.
- Events:
- Rapid formation of secondary structure.
- Formation of domains through cooperative aggregation (concept of ‘folding nuclei’).
- Formation of assembled domains (concept of ‘molten globule’)
- Adjustment of conformation.
- More rigid structure.

Describe the essential fatty acids.
Essential Fatty Acids
- Humans cannot synthesize ω-3 and ω-6 fatty acids.
- Need to ingest these via diet or their precursors such as:
- Linoleic acid (18:2 ω6)
- Linolenic acid (18:3 ω3)
- Precursors termed Essential Fatty Acids.
- Linoleic acid used to make arachidonic acid (20:4 ω6), a precursor for eicosanoids (prostaglandins, leukotrienes, and thromboxanes).
- Linolenic acid used to make eicosapentanoic acid (EPA) (20:5 ω3) and docosahexanoic acid (DHA) (22:6 ω3).
- Benefits:
- Immune system
- Cardiovascular system
- Nervous system
- Vision
- Cell membrane
Describe the essential monosaccharides.
Monosaccharides - Essential
- Essential because we do not produce enough to keep up with demand.

Describe the fates of 3-phosphoglycerate in AA synthesis.
AA Synthesis - Fates of 3-Phosphoglycerate
- Three reactions to get to Serine.
- With a little help from Vitamins B6 and B12, the side chain is removed from Serine, leaving behind Glycine.
- In humans, Serine is converted to Cysteine through a homocysteine intermediate.

Describe the fates of aspartate in AA synthesis.
AA Synthesis - Fates of Aspartate
- Glutamine donates an NH3 to Aspartate to form Asparagine.
- In other organisms, Aspartate can be modified to create Lysine, Methionine, and Threonine. These reactions are tightly regulated by feedback inhibition.
- Last image is pathways in other organisms.
- Understand the feedback regulation.

Describe the fates of glutamate in AA synthesis.
AA Synthesis - Fates of Glutamate
- Glutamine is formed in cells as a way to transport free NH4+ to the liver.
- Formed by glutamine sythetase (-etase = uses ATP).
- After a couple reductions and a cyclization, Proline is formed.
- After one reduction and a transamination, Ornithine is formed.
- Remember, ornithine is Arginine minus urea.
- Orinithine feeds back through the urea cycle to get back to argenine.
- Because argenine is used in urea cycle, we need to supplement argenine.

Describe the Fe-S clusters.
ETC - Fe-S Clusters
- Iron sulfur clusters present in iron sulfur proteins (aka non-heme iron proteins).
- Play role in reduction reactions.
- Exist in various configurations.
- Undergo oxidation-reduction reactions but the protons never leave the protein.
- Example: NADH-Q-oxidoreductase (Complex-I) Contains both 2Fe-2S and 4Fe-4S clusters.

Describe the form of glycerol and TAGs.
Glycerol & TAGs

Describe the general regulation of glycogen metabolism.
Glycogen Metabolism - General Regulation
-
Glycogenesis favored in fed state:
- Blood glucose high.
- Insulin high.
- Cellular ATP high (signal of high energy).
- Predominant states:
- Dephospho form of glycogen synthase (active).
- Dephospho form of glycogen phosphorylase (inactive).
-
Glycogenolysis favored in:
-
Fasting state:
- Blood glucose low.
- Glucagon high.
-
Exercise:
- Cellular calcium high (in exercising muscles).
- AMP high (from breakdown of ATP)
- Predominant states:
- Phospho form of glycogen synthase (inactive).
- Phospho form of glycogen phosphorylase (active).
-
Fasting state:
Describe the glucose 6-phosphatase step of glycogenolysis.
Glycogenolysis - Glucose 6-Phosphatase
- Gluc-6-phosphate cannot get out of the cell.
- Only the liver has glucose 6-phosphatase.
- Converts it to glucose which can be released into blood stream.
- Muscle cells do not have glucose 6-phosphatase so Gluc-6-phosphate stays.

Describe the Hb/oxygen dissociation curve.
Oxygen Dissociation Curve
- Steepest part of curve corresponds to drop in pressure from 40 torr (resting tissues) and 20 torr (exercising tissues).
- Hb is very effective in providing oxygen to exercising tissues.
- Sigmoidal shape is the result of the interactions between the globin subunits (cooperativity).

Describe the hydrogen bond.
Electrostatic Interactions - Hydrogen Bond

Describe the hydrophobic amino acids.
Amino Acids - Hydrophobic
- Non-polar
- Side chains are hydrocarbon structures.

Describe the induced fit model.
Enzymes - Induced Fit Model
- Enzyme changes shape as substrate binds to force substrate into transition state.

Describe the intermediate molecules, produced by 3 different pathways, that are used in the synthesis of AA.
AA Synthesis - Precursors / Intermediates
- Intermediate molecules from 3 pathways are used to synthesize amino acids.
- Glycolysis:
- 3-phosphoglycerate
- Phosphoenolpyruvate
- Pyruvate
- TCA cycle:
- α-ketoglutarate
- Oxaloacetate
- PPP:
- Ribose-5-phosphate
- Erythrose-4-phosphate

Describe the mobile e- carriers of the ETC.
ETC - Mobile e- Carriers
- Coenzyme Q aka ubiquinone:
- Transfers electrons from NADH Q oxidoreductase and the Succinate Q reductase to Q cytochrome c oxidoreductase.
- Coenzyme Q has long tail made of 5-C isoprene units which makes it hydrophobic.
- Most common is CoQ 10.
- Cytochrome c:
- Shuttles electrons from Q Cytochrome c oxidoreductase to cytochrome c oxidase.
- Final component of the ETC.
- Catalyzes reduction of O2.

Describe the nitrogenous bases of nucleic acids.
Nucleic Acids - Nitrogenous Base
- 2 types:
- Purines:
- Attaches to sugar by an N-glycosidic linkage between N-9 of purine and C-1’ of sugar.
- Pyrimidines
- Attaches to sugar by an N-glycosidic linkage between N-1 of pyrimidine and C-1’ of the sugar.
*
- Attaches to sugar by an N-glycosidic linkage between N-1 of pyrimidine and C-1’ of the sugar.
- Purines:

Describe the nonoxidative phase of the pentose phosphate pathway.
Pentose Phosphate Pathway - Nonoxidative Phase
- Catalyzes the interconversion of three-, four-, five-, six-, and seven-carbon sugars in a series of nonoxidative reactions.
- Excess five-carbon sugars may be converted into intermediates of the glycolytic pathway.
- 4 “shuffling” events:
- Ribulose 5-phosphate isomerization by:
- Ribose-5-phosphate isomerase –> Ribose-5-Phosphate (= a tautomer).
- Ribulose-5-phosphate epimerase –> Xylulose-5-Phosphate (= an epimer).
-
Transketolase transfers 2-C from Xylulose-5-phosphate (5-C) , leaving behind Glyceraldehyde-3-phosphate (3-C):
- Added to Ribose-5-phosphate (5-C) makes Sedoheptulose-7-phosphate (7-C).
- Added to Erythrose-4-phosphate (4-C) makes Fructose-6-phosphate (6-C).
- Transition state stabilized by TPP coenzyme (Vitamin B1).
-
Transaldolase transfers 3-C units:
- From Sedoheptulose-7-phosphate (7-C), leaving behind Erythrose-4-phosphate (4-C) (makes Transketolase substrate).
- Onto Glyceraldehyde-3-phosphate (3-C), making Fructose-6-phosphate (6-C) (makes same product as Transketolase).
- Transition state stabilized by Lys sidechain.
- Regeneration of Glucose-6-Phosphate:
- Utilizes gluconeogenesis pathway.
- Ribulose 5-phosphate isomerization by:

Describe the oxidative phase of the pentose phosphate pathway.
Pentose Phosphate Pathway - Oxidatvie Phase
- 3 main reactions:
-
Glucose 6-Phosphate Dehydrogenase
- Oxidizes Glucose 6-Phosphate to form a lactone.
- Reduces NADP+ to form NADPH.
- Rate-limiting reaction.
- Lactonase is a Hydrolase! (Opens the ring by adding water).
-
6-Phosphogluconate Dehydrogenase:
- Oxidative Decarboxylation of 6-Phosphogluconate to form Ribulose 5-phosphate.
- Reduces NADP+ to form NADPH.
-
Glucose 6-Phosphate Dehydrogenase

Describe the pathway integration of fatty acid metabolism.
Fatty Acid Metabolism - Pathway Integration

Describe the pentose phosphate pathway (PPP).
Pentose Phosphate Pathway
- Occurs in cytoplasm.
- Crucial source of NADPH for reductive biosynthesis and protection against oxidative stress.
- 2 phases:
- Phase I - oxidative phase:
- Processes G6P –> ribose 5-phosphate.
- Produces: 2 NADPH and CO2.
- Phase II - nonoxidative phase:
- Catalyzes the interconversion of three-, four-, five-, six-, and seven-carbon sugars in a series of nonoxidative reactions.
- Excess five-carbon sugars may be converted into intermediates of the glycolytic pathway.
- Phase I - oxidative phase:

Describe the peptide bond.
Peptide Bond
- Bond between the amine of one amino acid and the carboxyl of another.
- Planar
- Some rotation can occur, depending on steric hinderances.
- Trans (side chains alternating up and down) is preferred to cis form due to steric strains from side chains.

Describe the phosphoglucomutase step in glycogenolysis.
Glycogenolysis - Phosphoglucomutase
- Converts Gluc-1-phosphate to Gluc-6-phosphate.
- A phosphoryl group is transferred from the enzyme to the substrate, and a different phosphoryl group is transferred back to restore the enzyme to its initial state.

Describe the polar amino acids.
Amino Acids - Polar
- Side chains have H-bonding potential:
- OH
- Amines

Describe the production of biosynthetic precursors by the citric acid cycle.
Citric Acid Cycle - Biosynthetic Precursor Production
- When energy needs are met, intermediates are drawn for biosynthesis of other molecules.
- They are replenished by formation of oxaloacetate from pyruvate.

Describe the reactions catalyzed by FAS.
FAS

Describe the reactions of gluconeogenesis.
Gluconeogenesis - Reactions

Describe the reactions that take place in fatty acid breakdown.
Fatty Acid Breakdown - Reactions

Describe the regulation of acetyl-CoA carboxylase.
Acetyl-CoA Carboxylase - Regulation
- Rate limiting step of FA synthesis!!!!
- ACC - Inactive dimer, active polymer.
- Allosteric regulation:
- Stimulated by citrate
- Inhibited by long chain fatty acids (palmitate).
- Phosphorylation (-) / Dephosphorylation (+)
- Insulin - stimulates ACC via activation of protein phosphatase.
- Epinephrine - inhibits ACC via activation of protein kinase A.
- Glucagon - inhibits ACC via activation of protein kinase A.
- AMP - inhibits ACC via activation of AMP kinase (energy sensor).
- Induction:
- Gene expression up-regulated by high carb/low fat diet.

Describe the regulation of ATP citrate lyase.
ATP Citrate Lyase - Regulation
- Stimulated by phosphorylation.
- Gene expression induced by glucose/insulin.
- Induction of gene expression counteracted by polyunsaturated fatty acids (PUFAs).
- Induction of gene expression counteracted by leptin.

Describe the regulation of FA synthesis.
Fatty Acid Synthesis - Regulation
- Multiple points of regulation.
- Gene expression of the enzymes induced by low fat, high carb diet.
- 25-100-fold more active in fed state.
- Enzymes:
- ATP citrate lyase (Phase I)
- Acetyl CoA Carboxylase (Phase II – rate limiting step).
- Fatty acid synthase (Phase III)
Describe the regulation of fatty acid synthase.
Fatty Acid Synthase - Regulation
- Allosteric effect (presence of phosphorylated sugars) - increase activity.
- Induction and repression at gene level:
- Insulin and glucocorticoid hormones increase synthesis.
- High carb/low fat diets increase synthesis.
- High fat diets as well as starvation lowers synthesis.
- High PUFA suppresses synthesis.

Describe the regulation of glutamine synthesis.
Glutamine Synthesis - Regulation
- Adenylylation is the addition of an AMP ribonucleotide.
- Adenylylation inactivates Glutamine synthetase.
- Uridylylation is the addition of a UMP ribonucleotide.
- Feedback inhibition:
- Glutamine maintains PII in its inhibitory form (-UMP).
- Feedforward activation:
- α-ketoglutarate and ATP maintain PII in its activating form (+UMP).

Describe the regulation of glycogen metabolism by insulin.
Glycogen Metabolism - Regulation by Insulin
- High blood glucose (promote glycogen synthesis).
- Release of insulin by β cells of pancreas.
- Binding of insulin to its receptor tyrosine kinase.
- Activation of signaling cascade.
-
Four key proteins involved:
- GLUT 4
- Protein kinase B (PKB)
- Protein phosphatase 1 (PP1)
- Glycogen synthase kinase 3 (GSK3)
- Formation of the insulin receptor complex.
- Activation of PKB.
- Translocation of GLUT4 to membrane.
- PKB phosphorylates PP1 (activate) and GSK3 (inactivate).
- Active PP1 dephosphorylates glycogen synthase (activate) and dephosphorylates glycogen phosphorylase (inactivate).
- Net result – glycogen synthesis via activation of glycogen synthase and inactivation of glycogen phosphorylase.

Describe the regulation of glycogen synthesis.
Glycogenesis - Regulation
- Key enzyme: glycogen synthase (GS):
- 2 forms:
- Active non-phosphorylated “a” form.
- Inactive phosphorylated “b” form.
- Interconversion mediated by covalent modification (fine-tuning role).
- 2 forms:
- Phosphorylated by several kinases most importantly glycogen synthase kinase (GSK).
- GSK under the control of insulin and PKA.
- Allosteric regulation:
- Gluc-6-phosphate powerful activator.
- Stabilizes active form.
Describe the regulation of glycogenolysis.
Glycogenolysis - Regulation
- Key enzyme: glycogen phosphorylase (GP):
- 2 forms:
- Active “a” form (R relaxed state) – in liver.
- Inactive “b” form (T tense state) – in muscle.
- Both isozymes exist in equilibrium between R and T.
- GP regulated by:
- Several allosteric effectors (signal energy state of the cell).
- Reversible phosphorylation (responsive to hormones).
- 2 forms:

Describe the regulation of glycolysis and gluconeogenesis in the liver.
Glycolysis and Gluconeogenesis - Regulation in the Liver
- In the liver, rates of glycolysis and gluconeogenesis are adjusted to maintain blood glucose.
- Fructose 2,6-bisphosphate strongly stimulates phosphofructokinase and inhibits fructose 1,6-bisphosphatase.
- Two enzymes regulate the concentration of fructose 2,6-bisphosphate:
- Phosphofructokinase 2 (PFK2)
- Fructose bisphosphatase 2 (FBPase2)

Describe the regulation of the citric acid cycle.
Citric Acid Cycle - Regulation
- PDH - Acetyl-CoA production:
- High [acetyl CoA] directly inhibits PDH complex subunit E2.
- Energy charge of cell dictates PDH complex activity.
- Phosphatases are also stimulated by Ca2+ which increases to initiate muscle contraction.
- Insulin can stimulate fatty acid synthesis by activating phosphatases and increasing the conversion of pyruvate to acetyl CoA (precursor for fatty acids).
- High [acetyl CoA] directly inhibits PDH complex subunit E2.
- Citrate synthase prevents the wasteful hydrolysis of acetylCoA.
- Oxaloacetate binds to the enzyme first, then enzyme undergoes the configurational changes to accept acetylCoA.
- Condensation reaction yields citrate.
- Isocitrate dehydrogenase:
- Allosterically stimulated by ADP, enhancing enzyme affinity for substrate.
- Reaction product, NADH also inhibits by directly displacing NAD+.
- α-ketogluturate dehydrogenase:
- Remember: this complex is similar to PDH.
- Also similar to PDH in regulation.
- Allosterically inhibited by its products, succinyl CoA and NADH.
- Control at these sites help integrate the TCA cycle with other pathways.
- Control at isocitrate dehydrogenase leads to build up of citrate (easily converted from isocitrate) which can transport to the cytosol and signal phosphofructokinase and halt glycolysis.
- Also, α-ketoglutarate that builds up from enzyme inhibition can be used for synthesis of amino acids and purine bases.

Describe the role of ACTase in metabolism.
Aspartate Carbamoyl-Transferase (ACTase)
- AKA: aspartate transcarbamoylase.
- One of the key enzymes for the regulation of pyrimidine biosynthesis.
- Catalyzes the first step in the biosynthesis of pyrimidines: the condensation of aspartate and carbamoyl phosphate to form N -carbamoylaspartate and orthophosphate.
- Inhibited by CTP the final product of the ATCase-initiated pathway (prefers the T/inactive state).
- An example of feedback inhibition (inhibition of enzyme by end-product of pathway).
- CTP binds to allosteric site.
- Activated by ATP (prefers the R/active state).

Describe the role of enzyme amount in regulating enzymes.
Enzyme Regulation - Enzyme Amount
- Protein synthesis regulation = ON/OFF switch.
- 2 levels of control are possible:
- Transcription regulation at promoters.
- Translation regulation at UTRs.
Describe the role of proteolytic activation in regulation of enzymes.
Enzyme Regulation - Proteolytic Activation
- Irreversible covalent modification.
- Many important enzymes begin life as zymogens:
- Proteases:
- Digestive enzymes
- Collagenase (development)
- Caspases (apoptosis)
- Collagen
- Blood clotting factors.
- Insulin/hormones
- Proteases:
Describe the role of reversible covalent modifications in regulation of enzymes.
Enzymes Regulation - Covalent Modifications
- Add “functional groups” to activate/inactivate the enzyme.
- Post-translational modifications create nonproteinogenic amino acids.
- Common additions:
- Lipids:
- Myristic acid -> farnesyl
- Nucleic acids:
- ADP-ribose
- Proteins:
- Ubiquitin
- Carbohydrates:
- Greatest source of diversity to the proteome.
- O- vs N-linkages (which atom on amino acid it links to).
- Composition of sugars.
- Branched vs. unbranched.
- Length of oligosaccharide.
- Small molecules:
- γ-carboxylation
- Sulfation
- Acetylation
- Methylation
- Phosphorylation
- Lipids:

Describe the shuttling of oxaloacetate (OAA).
Shuttling of Oxaloacetate (OAA)
- PC is a mitochondrial enzyme.
- Other enzymes of gluconeogenesis found in cytoplasm.
- OAA (product of PC) transported to cytoplasm via malate shuttle.

Describe the sugar component of nucleic acids.
Nucleic Acids - Sugar
- 2 types:
- Ribose
- Deoxyribose
- Base attaches by glycosidic bond to the 1’ - C.
- Presence of O on 2’ - C determines ribose or deoxyribose.
- Phosphate attaches by phosphodiester bond to 3’ - C.

Describe the synthesis of aromatic AA.
AA Synthesis - Aromatic AAs
- Aromatic amino acids are derived from intermediates originating in the Pentose Phosphate Pathway.
- Histidine is synthesized from ribose-5-phosphate.
- Tryptophan, Phenylalanine, and Tyrosine synthesis begins by combining Erythrose-4-phosphate and phosphoenolpyruvate to make Chorismate.

Describe the synthesis of the branched chain amino acids.
AA Synthesis - Branch Chain Amino Acids
- The branched amino acids Valine, Leucine, and Isoleucine are synthesized from pyruvate.
- Not done in humans.
- These three amino acids:
- Have complex syntheses.
- Only exist to be incorporated into proteins.
- Syntheses are regulated by feedback inhibition.

Describe the synthesis of Tyrosine.
AA Synthesis - Tyrosine
- Tyrosine is a catabolic intermediate of Phenylalanine.
- More than one pathway, however, making from Phe is one step and done.

Describe the thermodynamics of a reaction.
Thermodynamics of a Reaction

Describe the transamination reactions in amino acid synthesis.
Amino Acid Synthesis - Transamination
- Pyruvate Alanine
- Exchange by SGPT.
- OAA Aspartate
- Exchange by SGOT.
- α-Ketoglutarate Glutamate
- Exchange by Glutamate Dehydrogenase.

Describe the types of facilitated diffusion.
Facilitated Diffusion - Channels
- Gap Junctions:
- Allow cytoplasm sharing.
- No specificity filter.
- Auquaporins:
- Let water but not ions through.
- Utilize adhesion and cohesion to facilitate movement of water.

Describe the urea cycle.
Urea Cycle
- NH4+ is a toxic byproduct of amino acid catabolism.
- NH4+ can be converted to urea in the liver.
- Urea is transported to the kidneys to be excreted from the body.
- Urea is composed of 4 pieces:
- 2 NH4+ - 1 from aspartate and 1 from carbamoyl phosphate.
- C from bicarbonate.
- O from water.
- Carbamoyl Phosphate Synthetase I (CPSI) combines CO2 and NH4 to make Carbamoyl Phosphate, using 2 ATP.
- Creation of Carbamoyl Phosphate is the committed step in the urea cycle.
- NAG (N-acetyl glutamate) is an allosteric activator of CPSI.
- Ornithine and Citrulline are nonproteinogenic amino acids.
- Ornithine must move into the mitochondria to combine with carbamoyl phosphate.
- Citrulline is exported to the cytoplasm.
- Aspartate donates an NH3 at the cost of 2 ATP-equivalents.
- Remainder of aspartate is fumarate!
- The amino acid Arginine is created.
- Fumarate –> OAA uses TCA cycle!
- OAA –> Asp conversion is performed by SGOT aminotransferase.
- Ornithine is created when urea is removed from Arginine.
- The addition of water when cleaving Arginine adds the final O of urea.
- Ornithine enters into the mitochondria and continues the cycle.
- Adult humans produce ~30g of urea each day, accounting for ~50% of ATP consumption in the liver.
- Aquatic animals can excrete ammonia directly, so amphibians only express urea cycle enzymes at metamorphosis.

Describe the utilization of acetoacetate.
Acetoacetate - Utilization

Describe transferases.
Enzymes - Transferases
- Move a functional group (group transfer).
- Activated carriers:
- Transfer phosphate group:
- ATP
- Pyridoxal phosphate
- Transfer methyl group:
- SAM
- Tetrahydrofolate
- 5’-deoxyadenosylcobalamin
- Transfer phosphate group:

Describe UMP synthetase.
UMP Synthetase
- Multi functional protein.
- Removes PPi when orotate is added to PRPP.
- Same PPi that was originally added when PRPP was made,
- Decarboxylates orotate to form uracil.
- Deficiency results in Hereditary Orotic Acidura.
- Symptoms:
- Excessive orotate excreted in urine.
- Megaloblastic anemia that fails to respond to B9/B12 treatments.
- Most commonly caused by B9 and/or B12 deficiencies.
- Symptoms:

Describe uncompetitive inhibition.
Inhibition - Uncompetitive.
- Binds to allosteric site.
- Can only bind to ES complex.
- Lineweaver-Burk plots for rate with and without inhibitor, are parallel.
- νmax is variable.
- Km is variable.

Describe uric acid metabolism.
Uric Acid Metabolism
- Final product of purine catabolism.
- Uric Acid, like glutathione, is an antioxidant.
- Uric Acid/Urate is insoluble.
- Urate Oxidase solubilizes urate, at the cost of its antioxidant potential.
- Humans and the great apes have mutated Urate Oxidase genes in order to take advantage of the antioxidant function.

Describe weak acid and base equilibrium.
Weak Acid and Base Equilibrium

Describe Michaelis-Menten enzymes as they relate to cooperativity.
Michaelis-Menten Enzymes - Cooperativity
- Micahelis-Menten enzymes may have multiple binding sites only if non-cooperative.
- Michaelis-Menten enzymes show hyperbolic curve on graph.
- Cooperative enzymes show sigmoidal curve on graph.

Describe NADPH.
NADPH
- Usually functions as a cofactor for reductases, enzymes that catalyze substrate reduction.
- PPP is primary source of NADPH.
- Used in biosynthesis of:
- FA
- Cholesterol
- Neurotransmitters
- Nucleotides
- Used in detoxification to reduce harmful oxidative species:
- Reduction of oxidized glutathione.
- Cytochrome P450 monooxygenases.
How are amino acids sorted by size/shape?
Amino Acids - Size/Shape
- Large - long and linear in shape.
- Very large - aromatics.

How are metabolic enzymes regulated?
Metabolic Enzymes - Regulation
- Compartmentalization (different locations).
- Enzyme concentration - on/off switch.
- Enzyme activity - volume control.
- Hormone signals and second messengers - master regulators.
List the anabolic pathways.
Anabolic Pathways
- Macromolecule synthesis.
- Storage polymers.

List the catabolic pathways.
Catabolic Pathways
- Energy production.
- Macromolecule recycling.

List the common monosaccharides and be able to identify and describe their structures.
Monosaccharides

What are buffers?
Buffers
- Weak acids or bases that can stabilize pH.

What are fisher projections?
Fisher Projections
- Linear versions of carbohydrates.
- Carbon numbering starts at the carbonyl end of the aldose.
- Know tautomers!

What are the 3 phases of FA synthesis?
Fatty Acid Synthesis - Phases
- Phase I: Cytosolic entry of Acetyl CoA
- Made in mitochondrial matrix but needed in cytoplasm.
- Phase II: Generation of Malonyl CoA.
- Acetyl CoA is carboxylated to malonyl CoA. Most important substrate in FA synthesis. Rate limiting reaction.
- Phase III: Fatty acid chain formation.
- The enzyme Fatty acid synthase catalyzes 7 reactions that incorporate Acetyl CoA and Malonyl CoA into palmitate, a C16 fatty acid.

What are the 3 types of isomers?
Isomers - Types
- Constitutional
- Configurational
- Conformational

What are the essential amino acids?
Essential Amino Acids
- Histidine
- Isoleucine
- Leucine
- Lysine
- Phenylalanine
- Threonine
- Tryptophan
- Valine
- (Arginine) - can make, but production can’t typically keep up with consumption.
- (Methionine) - can make, but production can’t typically keep up with consumption.
What are the key points about the citric acid cycle?
Citric Acid Cycle - Key Points
- All energy containing nutrients should be converted to Acetyl CoA.
- The citric acid cycle occurs under aerobic conditions and produces more energy from glucose than glycolysis.
- The citric acid cycle takes place in the mitochondria.
- Pyruvate dehydrogenase (PDH) links glycolysis to the citric acid cycle.
- PDH contains 3 enzymes and uses 5 cofactors to convert Pyruvate —> Acetyl CoA for entry into the citric acid cycle.
- The citric acid cycle oxidizes 2 carbon units.
- Entry to the citric acid cycle and metabolism through it are highly controlled.
- PDH (or PDC) is regulated both allosterically and by phosphorylation.
- The citric acid cycle is a source of biosynthetic precursors.
- Anaplerotic reactions are required during states of low energy.
What are the methods of crossing a membrane.
Lipid Bilayer - Transport Methods
- Passive Transport:
- No energy needed.
- Solute travels down concentration gradient.
- Active Transport:
- Coupled to ATP hydrolysis.
- Solute travels against concentration gradient.
- Secondary active transporters - use gradients established by active transporters to drive transport.
- Antiporter
- Symporter
- Uniporter

What are the nonessential amino acids?
Nonessential Amino Acids
- Only 10 of the 20 proteinogenic amino acids are synthesized de novo in humans.
- Alanine
- Asparagine
- Aspartic Acid
- Cysteine
- Glutamic Acid
- Glutamine
- Glycine
- Proline
- Serine
- Tyrosine
What are the sources of NADPH for FA synthesis?
Fatty Acid Synthesis - Sources of NADPH
- Malic enzyme: 1
- Pentose phosphate pathway: 2 - 12
What are the sources of triacylglycerol?
TAG - Sources
- Dietary TAG (processed in intestinal cells).
- De Novo TAG (in hepatocytes).
- De Novo TAG (in adipocytes).
What are the stages of glycolysis?
Glycolysis - Stages
- Occurs in the cytoplasm of eukaryotic cells.
- Divided into two stages:
- Trapping of glucose and its cleavage into 2 interconvertible 3-carbon molecules.
- Generation of ATP.

What are the three general categories of transporters?
Transporters - Categories
- Pumps perform primary active transport.
- A water pump FIGHTS GRAVITY and actively pulls water to the house.
- Carriers traverse the membrane without needing (extra) energy.
- Carrying a STOWAWAY along for the ride.
- Channels are used in passive transport.
- The channel opened and allowed your ship to float gently out of the GRIDLOCK from behind the dam.

What are the types of electrostatic interactions?
Electrostatic Interactions - Types
- Charge-charge
- Charge-dipole
- Dipole-dipole
- Charge-induced dipole
- Dipole-induced dipole
- Dispersion (van der Waals)
- Hydrogen bonds

What do enzymes do?
Enzymes - Catalysis
- Enzymes do:
- Lower activation energy.
- Stabilize transition state.
- Enzymes do not:
- Change the ΔG of the reaction.
- Irreversibly change shape.
What is a nucleic acid?
Nucleic Acid
- Consists of:
- Phosphate group.
- Sugar group.
- Nitrogenous base.
- 2 types:
- DNA:
- Deoxyribonucleic acid
- 2’-C lacks O.
- RNA:
- Ribonucleic acid
- 2’-C has O.
- DNA:

What is a nucleoside?
Nucleic Acids - Nucleosides
- Contains a nitrogenous base bonded to a sugar.
- Four nucleosides in RNA:
- Adenosine
- Guanosine
- Cytidine
- Uridine
- Four nucleosides in DNA:
- Deoxyadenosine
- Deoxyguanosine
- Deoxycytidine
- Thymidine

What is a nucleotide?
Nucleic Acids - Nucleotide
- A nucleoside (base and sugar) joined to one or more phosphoryl groups by an ester linkage.
- Nucleotide triphosphates are the building blocks (monomers) that are linked to form RNA and DNA.
- 4 nucleotide monophosphates:
- Deoxyadenylate
- Deoxyguanylate
- Deoxycytidylate
- Thymidate (no deoxy because rarely found in RNA)

What is a phosphodiester bond?
Covalent Bonds - Phosphodiester
- Bond between nucleotides.
- Found in the backbone of DNA and RNA.

What is catalysis.
A modification and especially increase in the rate of a chemical reaction induced by material unchanged chemically at the end of the reaction.
What is feedback control?

What is kcat/Km?
kcat/Km
- Specificity constant - measure of enzyme performance by predicting the fate of ES.
- Most important constant.
- Used in virtually all literature.
- Good enzyme:
- ES goes to product.
- kcat/Km ~ k1
-
Bad enzyme:
- ES goes to reactants.
- kcat/Km ~ 1/Km

What is kcat?
kcat
- Turnover number - number of substrate molecules converted per active site per time (first-order rate constant).
- Assuming steady state:
- When [S] >> [E], ΔS~0
- Formation of ES occurs at the same rate as its loss (in either direction).
- k2 is the rate-determining step.
- kcat can be defined.

What is Km?
Km
- Michaelis Constant
- Dissociation constant for ES.
- [S] where reaction rate is 1/2 νmax or 1/2 of the active sites are full.
- Assuming steady state:
- When [S] >> [E], ΔS~0
- Formation of ES occurs at the same rate as its loss (in either direction).
- Can define Km.

What is KS?
KS
- Dissociation constant for substrate binding.
- Can be defined for a reaction assuming that binding of S is at equilibrium.
- At equilibrium KS = KD

What is PII?
PII
- A trimeric protein that can exist in two forms, unmodified (PII) or covalently bound to UMP (PII-UMP).
- The complex of PII and adenylyl transferase catalyzes the attachment of an AMP unit to glutamine synthetase, which reduces its activity.
- Conversely, the complex of PII-UMP and adenylyl transferase removes AMP from the adenylylated enzyme.
- PII regulation:
- PII is converted into PII-UMP by the attachment of uridine monophosphate to a specific tyrosine residue.
- Stimulated by ATP and α-ketoglutarate.
- Inhibited by glutamine.
- UMP units on PII are removed by hydrolysis.
- Promoted by glutamine.
- Inhibited by α-ketoglutarate.
- Catalyzed by uridylyl transferase.
- PII is converted into PII-UMP by the attachment of uridine monophosphate to a specific tyrosine residue.

What is substrate-level control?

What is the citric acid cycle.
Citric Acid Cycle
- AKA: TCA & Krebs
- A biochemical hub of the intermediary metabolism:
- Oxidizing carbon fuels for harvesting high energy electrons (e-).
- It is amphibolic (i.e., catabolism and anabolism).
- Source of precursors for biosynthesis.
- Takes place inside mitochondria.
- Carries out the oxidation of 2-carbon units to produce:
- 2 - CO2 molecules
- 1 - GTP
- High energy electrons in the form of NADH and FADH2.

What is the end product of FA synthesis?
Palmitic acid.

What is the equation for pKa & pKb?
pKa & pKb

What is the equation for Ka & Kb?
Dissociation Constant - Ka & Kb

What is the Henderson-Hasselbalch equation and what does it mean?
Henderson-Hasselbalch
- An equation used to analyze the effect of the buffer in quantitative terms.
- pH < pKa - most molecules are protonated since [HA]>[A-]
- pH > pKa - most molecules are deprotonated since [HA] < [A-]
- pH = pKa - molecules are just as likely to be protonated as deprotonated.
- Note: HA and A- DO NOT indicate molecule charge.

What is the major source of carbon for FA synthesis?
Dietary carbohydrates.
What is the precursor for all nucleotides?
PRPP
- 5-Phospho-α-D-ribosyl-1-pyrophosphate
- Activated form of ribose phosphate.
- Important precursor for all nucleotides.

What is νmax?
νmax
- Maximum velocity - maximum rate possible for a given concentration of enzyme.
- Reached when enzyme is fully saturated:
- [ES] = [E]T
- Thus ν0 = kcat[ES] becomes νmax=kcat[E]T.

What tissues have active pentose phosphate pathways?
Tissues with Active PPPs

Where does FA synthesis occur?
Fatty Acid Synthesis
- Occurs primarily in the liver, but also occurs in adipose tissue, brain, kidneys, and lactating mammary glands.
Why is the citric acid cycle called anaplerotic?
Citric Acid Cycle - Anaplerotic
- Anaplerotic “fill up” reactions provide intermediates for replenish TCA Cycle.
- Two major anaplerotic reactions:
- Degradation of amino acids.
- Carboxylation of Pyruvate.



