Antineoplastics Flashcards
Mechanism for Cyclophosphamide
Cyclophosphamide (prodrug) is bioactivated in the liver by CYP2B6, producing acrolein –> MESNA also given to detoxify in bladder
The phosphoramide mustard produced by the bioactivation of cyclophosphamide alkylates guanine residues in DNA
DNA can become crosslinked, impairing replication

Ifosfamide mechanism
Ifosfamide (prodrug) is bioactivated by CYP3A4
Bioactivation of ifosfamide creates acrolein and a unique byproduct, chloroacetaldehyde, which is both neurotoxic and nephrotoxic
Reacts with DNA similarly to cyclophosphamide – crosslinking
Carmustine (and other nitrosoureas) mechanism
Nitrosoureas are lipid soluble and have excellent absorption into cerebral spinal fluid and therefore are routinely used for brain cancers
Carmustine is chemically unstable and metabolized quickly by the liver
Carmustine is bifunctional reacting with N7 of guanine, causing DNA crosslinks
Use of busulfan
pre-conditioning regime for hematopoietic stem cell transplant because of its profound bone marrow suppressio
Busulfan mechanism
Busulfan is subject to phase II detoxification through glutathione-S-transferase. CYP enzymes play a role in late stages of busulfan metabolism and elimination.
Busulfan is bifunctional as N7 of guanines attack carbons adjacent to sulfonate groups thereby causing DNA crosslinks
Temozolamide mechanism
Temozolamide undergoes a series of spontaneous transformations at physiological pH forming MTIC, which then releases a methyl diazonium ion, the active methylating agent.
NOT by p450, but dacarbazine is
Temozolamide and dacarbazine alkylate DNA by methylation of O6 and N7 atoms of guanine residues
Induced damage can be repaired by MGMT
Cisplatin/carboplatin mechanism
Inside the body, the chloride groups of cisplatin undergo displacement reactions first with water and then with N7 groups on guanine residues
Modifications to the drug by replacing the chloride ligands with cyclobutane dicarboxylic acid forming carboplatin, further decrease reactivity thereby reducing most side effects
Works by crosslinking to DNA, then expelled with urine


Allopurinol and azathioprine
Allopurinol (a xanthine oxidase inhibitor) is often given to patients receiving chemotherapy to ameliorate symptoms of gout related to tumor lysis syndrome.
Allopurinol should not be given to patients receiving 6-mercaptopurine.
Allopurinol will prevent the detoxification of 6-mercaptopurine to thiouric acid, thereby enhancing cytotoxicity.
What are the deoxy-adenosine analogs
Fludarabine, clofarabine and cladribine
Mechanism for Fludarabine and clofarabine
The reverse orientation of the OH group at the 2’ position in arabinose relative to ribose inhibits ribonucleotide reductase and blocks the production of dNTPs needed for replication.
Fludarabine and clofarabine have additional effects on replication by inhibiting DNA polymerase.
Have to be di and triphosphorylated on their 5’ hydroxyl groups by mono and diphosphokinases for biological activity

Mechanism of cladribine
It inhibits both adenosine deaminase and ribonucleotide reductase
The build up of dATP in lymphocytes deficient in adenosine deaminase inhibits ribonucleotide reductase blocking dNTP synthesis and interfering with the proliferation of B and T cells
Requires bioactivation by mono and diphosphokinases
Cytarabine mechanism
Inhibits DNA polymerase
What is the main target of 5-fluorouracil
thymidylate synthase
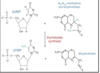
Drugs given alongside 5-fuorouracil to improve efficacy
Leucovorin, 5-formyl-tetrahydrofolate
Mechanism for 5-fluorouracil
Inhibition of thymidylate synthesis ultimately inhibits DNA replication thereby promoting apoptosis in proliferating cells
The rate-limiting step in 5-fluorouracil metabolism is catalyzed by dihydropyrimidine dehydrogenase which converts the drug to an inactive metabolite
Methotrexate mechanism
Methotrexate is an analog of dihydrofolate and is a competitive inhibitor of dihydrofolate reductase, so blocks the recycling of dihydrofolate to tetrahydrofolate
Methotrexate selectively kills proliferating cells because of their high demand for dTMP synthesis and consequently, dihydrofolate reductase activity
Mechanism for anthracyclins
Cycling back and forth between semi-quinone and quinone species generates reactive oxygen species which are important for the anti neoplastic activity of the anthracyclins.
The reactive oxygen species caused by redox cycling are also responsible for the adverse effects observed with anthracyclins, particularly their cardiotoxicity (dilated cardiomyopathy).

Besides forming reactive oxygen species, how else do anthracyclins work
intercalation of anthracyclins into DNA can interfere with replication contributing to their antineoplastic activity
anthracyclins can cause DNA damage by inhibiting topoisomerase enzymes
Bleomycin mechanism
Bleomycin is a glycopeptide that induces strand breakage in DNA
Bleomycin requires oxygen and divalent metals like iron for strand scission
How do topoisomerase enzymes work
Topoisomerase enzymes relieve this torsional stress by cleaving DNA strands and either taking out excess DNA turns (in front of the replication fork) or re-introducing DNA turns (behind the replication fork) to maintain the standard number of turns in normal B form DNA

Topoisomerase inhibitors broad mechanism
Topoisomerase inhibitors often trap topoisomerase enzymes in this covalent complex with DNA forming single-stand and double-strand breaks in DNA. Single- and double-stranded breaks in DNA are powerful signals to tumor suppressors like p53 promote cell cycle arrest to fix the damage prior to replicating the DNA, or if the damage is too great to promote apoptosis.

Podophyllotoxin (type of topoisomerase inhibitor) mechanism
binds to tubulin and poisoning mitotic spindles
etoposide and teniposide mechanism
interact with DNA and exert their effects via inhibition of topoisomerase II, inducing double -strand breaks in DNA


