Airway/Breathing Flashcards
Orotracheal Intubation Medical Directive CLINICAL CONSIDERATIONS (3)
Definition of intubation attempt: introducing the laryngoscope into the patient’s mouth with the intent to then insert an endotracheal tube is considered an attempt and should be documented as such including sucess or failure.
The number of attempts is clearly definded as two (2) intubation attempts per patient regardless of the route chosen.
Lidocaine adminstration prior to intubating a head injured patient is not indicated and has been removed.
Orotracheal Intubation Medical Directive INDICATIONS
Need for ventilatory assistance or airway control AND Other airway management is ineffective.
Orotracheal Intubation Medical Directive CONDITIONS

Orotracheal Intubation Medical Directive CONTRAINDICATIONS

Orotracheal Intubation Medical Directive TREATMENT

Orotracheal Intubation Medical Directive TREATMENT (2)

Orotracheal Intubation Medical Directive TREATMENT (3)

Orotracheal Intubation Medical Directive CLINICAL CONSIDERATIONS
An intubation attempt is defined as insertion of the laryngoscope blade into the mouth for the purposes of intubation.
Confirmation of orotracheal intubation must use ETCO2 (Waveform capnography). If waveform capnography is not availiable or not working then at least 3 secondary methods must be used. Additional secondary ETT placement confirmation devices may be auhtorized by the local medical director.
ETT placement must be reconfirmed immediately after every patient movement.
Orotracheal Intubation Medical Directive CLINICAL CONSIDERATIONS (1)
ETI (Endotracheal Intubation) is not mandatory. The importance of definitive airway management has given way to basic airway manegment and less invasive approaches.
The contraindication which references age < 50 refers specifically to paitents experiencing as asthma exacerbating and who are NOT in or near cardiac arrest.
Lidocaine spray is indicated for “awake” intubations only and should be applied to the hypoharynx.
Topical Lidocaine dosing has been updating: A single spray is 10mg, and the maximum body dose is 5 mg/kg which includes Lidocaine administered by any route (IV and topical).
Orotracheal Intubation Medical Directive CLINICAL CONSIDERATIONS (2)
In the treatment statement, “consider intubation” is followed by “with or without faciliatation devices”. This is a generic statement to address everything from the air trach, to the bougie to all things as yet undefined. The generic statement enables us to continue to use the directives despite changes in technology without being prescriptive.
ETI confrimation has been updated and now requires ETCO2 waveform capnograpghy as the only primary method. If it the most reliable method to monitor placement of an advanced airway. In the event it is not available, three (3) secondary methods must be used; for example: colormetric detector that changes color with exposure to CO2
Orotracheal Intubation Medical Directive
COMPANION DOCUMENT
ETI (Endotracheal Intubation) is not mandatory. The importance of definitive airway management has given way to basic airway management and less invasive appraoches.
The contraindication which references age < 50 refers specifically to patients experiencing as asthma exacerbation and who are NOT in or near cardiac arrest.
Lidocaine spray is indicated for “awake” intubations only and should be applied to the hypopharynx.
Topical Lidocaine dosing has been updated: A single spray is 10 mg, and the maximum body dose is 5 mg/kg which includes Lidocaine adminstered by any route (IV and topical)
In the treatment statement, “consider intubation” is followed by “with or without facilitation devices”. This is a generic statement to address everything from the air tach, to the bougie to all things as yet undefined. The generic statement enables us to continue to use the directives despite changes in technology without being prescriptive.
ETI confirmation has been updated and now requires ETCO2 waveform capnography as the only primary method. It is the most reliable method to monitor placement of an advanced airway. In the event it is not available, three (3) secondary methods must be used; for example: colormetric detector that changes color with exposure to CO2
Definition of intubation attempt: Introducing the laryngoscope into the patient’s mouth with the intent to then insert an endotracheal tube is considered an attempt and should be documented as such including success or failure.
The number of attempts is clearly defined as two (2) intubation attempts per patient regardless of the route chosen
Lidocaine administration prior to intubating a head injured patient is not indicated and has been removed.
Nasotracheal Intubation Medical Directive - AUX
INDICATIONS
Need for ventilatory assistance OR airway control;
AND
Other airway management is ineffective
Nasotracheal Intubation Medical Directive - AUX
CONDITIONS

Nasotracheal Intubation Medical Directive - AUX
CONTRAINDICATIONS

Nasotracheal Intubation Medical Directive - AUX
TREATMENT (1)
5Rs - Pt. - Drug - Dose - Route - Time

Nasotracheal Intubation Medical Directive - AUX
TREATMENT (2)
5Rs - Pt. - Drug - Dose - Route - Time

Nasotracheal Intubation Medical Directive - AUX
TREATMENT (3)

Nasotracheal Intubation Medical Directive
Clincial Considerations
A nasotracheal intubation attempt is defined as insertion of the nasotracheal tube into a nare.
Confrimation of nasotracheal placement must use ETCO2 (Waveform capnography). If wave-form capnography is not available or not working, then at least 2 secondary methods must be used.
ETT placement must be confirmed immediately after every patient movement.
Nasotracheal Intubation Medical Directive
COMPANION DOCUMENT
The contraindication which references age < 50 refers specifically to patients experiencing as asthma exacerbation and who are NOT in or near cardiac arrest.
NTI should only be attempted when deemed necessary and is reserved only for the “spontaneously breathing” patient in severe respiratory distress.
Lidocaine spray is indicated for “awake” intubations only and should be administered to both nares and the hypopharynx.
Topical Lidocaine dosing has been updated: A single spray is 10 mg, and the maximum body dose is 5 mg/kg which includes Lidocaine adminstered by any route (IV and topical)
NTI confirmation has been updated and now requires ETCO2 waveform capnography as the only primary method. It is the most reliable method to monitor placement of an advanced airway. In the event it is not available, two (2) secondary methods must be used; for example: colormetric detector that changes color with exposure to CO2
Definition of intubation attempt: Insertion into a nare is considered one attempt and should be documented as such including success or failure.
The number of attempts is clearly defined as two (2) intubation attempts per patient regardless of the route chosen
Supraglottic Airway Medical Directive - AUX
INDICATIONS
Need for ventilatory assistance OR airway control
AND
Other airway management is ineffective
Supraglottic Airway Medical Directive - AUX
CONDITIONS

Supraglottic Airway Medical Directive - AUX
CONTRAINDICATIONS

Supraglottic Airway Medical Directive - AUX
TREATMENT
5 Rs

Supraglottic Airway Medical Directive - AUX
CLINICAL CONSIDERATIONS
An attempt as supraglottic airway insertion is defined as the insertion of the supraglottic airway into the mouth.
Confirmation of supraglottic airway must use ETCO2 (Waveform capnography). If waveform capnograpghy is not available or is not working, then at least 2 secondary methods must be used.
Supraglottic Airway Medical Directive - AUX
COMPAINION DOCUMENT
Active Vomiting Defined:
Active vomiting is considered ongoing where the Paramedic is unable to clear the airway. In this situation, the supraglottic airway (SGA) should not be inserted.
If the patient has vomited, and the airway has been cleared successfully, a supraglottic airway may be inserted
The number of attempts is clearly defined as two (2) total per patient, and not per provider.
Confirmation of SGA insertion requires ETCO2 waveform capnography. It is the most reliable method to monitor placement of an advanced airway. If it is not available, at least two (2) secondary methods must be used. SGA placement should be verified frequently and again at transfer of care. Findings and witness (where possible) should be documented on the patient care record.
ROSC:
In the event the patient with a SGA in place sustains a ROSC, the SGA should only be removed if the gag reflex is stimulated or the patient begins to vomit; expect to remove it as the level of awareness improves.
Bronchoconstriction Medical Directive
INDICATIONS
Respiratory distress;
AND
Suspected bronchoconstriction
Bronchoconstriction Medical Directive
CONDITIONS

Bronchoconstriction Medical Directive
CONTRAINDICATIONS

Bronchoconstriction Medical Directive
TREATMENT (1)

Bronchoconstriction Medical Directive
TREATMENT (2)

Bronchoconstriction Medical Directive
CLINICAL CONSIDERATIONS
Epinephrine should be the 1st medication administered if the patient is apneic. Salbutamol MDI may be administered subquently using a BVM MDI adapter.
Nebulization is contraindicated in patients with a known or suspected fever or in the setting of a declared febrile respiratory illness outbreak by the local medical officer of health.
When administering salbutamol MDI, the rate of administration should be 100 mcg approximately every 4 breaths.
Bronchoconstriction Medical Directive
COMPANION DOCUMENT
Suspected bronchoconstriction applies to asthma, COPD, and other causes of bronchoconstriction. Symptoms of bronchoconstriction may include wheezing, coughing, dyspnea, decreased air entry and silent chest.
Epinephrine 1 : 1000 ( 1 mg/ml) IM is indicated when the patient is asthmatic and BVM ventilation is required. This is typically after salbutamol has had no effect, however salbutamol could be bypassed and epinephrine be administered immediately due to the severity of the patient’s condition. The indications to administer epinephrine do not change based on the ability to administer salbutamol.
When a dose of MDI salbutamol is administered, the intent is to deliver all six (6) (pediatric) or eight (8) (adult) sprays to complete a dose. It would be under unusual circumstances to deliver less than the full dose.
MDI administration is preffered over nebulization. If the patient is unable to accept or cooperate with MDI administration, the nebulized route may be considered (maximum three (3) doses).
Technique for administration of MDI salbutamol: Provide one MDI spray, followed by 4 breaths to allow for inhalation. It will take 1 minute to deliver a full adult dose to a patient breathing at a rate 32 breaths per minute.
The MDI should be considered a single patient use device.
Nebulization increases the mobilization of any contagion and a Paramedic should use PPE.
Bronchoconstriction Medical Directive
EPI DOSING CHART

Moderate to Severe Allergic Reaction
INDICATIONS
Exposure to a probable allergen
AND
Signs and/or symptoms of a moderate to severe allergic reaction (including anaphylaxis)
Moderate to Severe Allergic Reaction
CONDITIONS

Moderate to Severe Allergic Reaction
CONTRAINDICATIONS

Moderate to Severe Allergic Reaction
TREATMENT (1)
5 R’s

Moderate to Severe Allergic Reaction
TREATMENT (2)
5 R’s
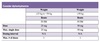
Moderate to Severe Allergic Reaction
CLINICAL CONSIDERATIONS
Epinephrine should be the first medication administered in anaphylaxis.
Moderate to Severe Allergic Reaction
COMPANION DOCUMENT (1)

Moderate to Severe Allergic Reaction
COMPANION DOCUMENT (2)

Croup Medical Directive
INDICATIONS
Severe respiratory distress
AND
Stridor at rest
AND
Current history of URTI;
AND
Barking cough OR recent history of a barking cough.
Croup Medical Directive
CONDITIONS

Croup Medical Directive
CONTRAINDICATIONS

Croup Medical Directive
TREATMENT

Croup Medical Directive
COMPANION DOCUMENT
The presentation must be severe. Most presentations of croup are mild and are well tolerated by the patient.
Prior to initiating nebulized epinephrine, moist/cold air may be attempted if available and patient’s condition permits.
Croup is occuring more and more frequently in older patients including adults, and is the indications are met, a patch to a BHP would be required to consider treatment under this medical directive.
All patients treated with epinephrine need to be transported for observation for rebound as the medication wears off.
Tension Pneumothorax Medical Directive
INDICATIONS
Suspected tension pneumothorax;
AND
Critically ill OR VSA;
AND
Absent or severely diminished breath sounds on the affected side(s)
Tension Pneumothorax Medical Directive
CONDITIONS

Tension Pneumothorax Medical Directive
CONTRAINDICATIONS

Tension Pneumothorax Medical Directive
TREATMENT

Tension Pneumothorax Medical Directive
CLINICAL CONSIDERATIONS
Needle thoracostomy may only be performed at the 2nd intercostal space in the midclavicular line.
Tension Pneumothorax Medical Directive
COMPANION DOCUMENT
Only the second inter-costal space is approved for chest needle placement for this reason: these patients are typically supine and/or spinal immobilized, and in that position, air rises and will escape at the second inter-costal space.
A one way valve should be applied to cover and protect the needle to allow air to escape from the chest.
Continuous Positive Airway Pressure (CPAP) - AUX
INDICATIONS
Severe respiratory distress
AND signs and/or symptoms of acute pulmonary edema OR COPD
Continuous Positive Airway Pressure (CPAP) - AUX
CONDITIONS

Continuous Positive Airway Pressure (CPAP) - AUX
CONTRAINDICATIONS

Continuous Positive Airway Pressure (CPAP) - AUX
TREATMENT
Confirm CPAP pressure by manometer (if available)

Continuous Positive Airway Pressure (CPAP) - AUX
CLINICAL CONSIDERATIONS
CPAP may be briefly interrupted to provide medications when necessary
The postiive pressure in the thorax may impede venticular filling resulating in decreased preload
Patients should be continuously monitored for signs of hypo-perfusion
Endotracheal and Tracheostomy Suctioning & Reinsertion
INDICATIONS
Patient with endotracheal or tracheostomy tube
AND
Airway obstruction or increased secretions.
Endotracheal and Tracheostomy Suctioning & Reinsertion
CONDITIONS

Endotracheal and Tracheostomy Suctioning & Reinsertion
CONTRAINDICATIONS

Endotracheal and Tracheostomy Suctioning & Reinsertion
TREATMENT

Endotracheal and Tracheostomy Suctioning & Reinsertion
CLINICAL CONSIDERATIONS



