Adrenal Flashcards
what part of the adrenal?

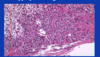
Largest zone is fasciculata and consists of large polygonal cells with prominent cytoplasmic borders. They are clear back heels which contain lipid, and when adrenal is functional and the lipid is depleted, the cells look more granular or eosinophilic.
What condition has cortical atrophy?
exogenous cushing
bromocriptine
dopaminergic agonist
high dose dexamethasone suppression test
cushing’s disease - where is it coming from?
higher dose of dexamethasone will decrease cortisol secretion in normal and patients with pituitary ACTH excess (cushin’g disease) but not in ectopic/adrenal ACTH cushing’s syndrome (there is much more acth in ectopic

what receptors do epi/NE bind?
- Epi and NE can either bind to either alpha-receptors or beta-receptors. There are several subtypes of each receptor.
- The important thing I want you to remember: The effect will depend on which receptor gets bound.
- Don’t memorize all this. Just realize the theme of hormone activity.
- Think of epinephrine as a hormone, whose activity depends on which receptor it binds.
- You can have totally opposite effects depending on the receptor.

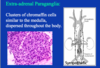
Conn syndrome
aldosterone producing adenoma - primary hyperaldosteronism
indistinguishable from other adenomas
small yellow circumscribed nodule, indstinguishable from others
cells mostly resemble fasiculata or mixed
chronic treatment of adrenal insufficiency
glucocorticoid/mineralocorticoid/androgen replacement
acute primary adrenal insufficiency
crisis in chronic insufficiency, withdrawl of corticosteroids (need to taper!!), DIC/post surgical/waterhouse-friederchsen
secondary adrenal insufficiency
pituitary/hypothalamic disorder that reduces ACTH –> decrease cortisol and androgens (similar to Addison’s disease)
diff: no hyperpigmentation (suppressed ACTH), normal aldo levels (not dep on ACTH)
cushing’s syndrome
syndrome of excess cortison from any cause
meds
cushing’s disease (pituitary tumor secreting ACTH)
ectopic ACTH secreting turmor
adrenal tumor (benign or malignant)
octreotide
somatostatin analog
waterhouse-friderichsen syndrome
crisis of acute adrenal insufficiency
overwhelming bacterial infection
DIC and rapid hypotension
massive adrenal hemorrhage and adrenocrotical insufficiency
Bacterial infection usually caused by meningitides bacteria
Because of all of that, they have massive adrenal hemorrhage and thus insufficiency.
What is the trigger for catecholamine release?
Acetylcholine
epinephrine and NE release

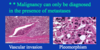
malignant pheochromocytoma
The only way we can know for sure if it’s malignant is if it’s metastasized. Otherwise they can show a lot of pleomorphism – see how big and dark these nuclei are. That’s not a helpful feature in endocrine organs as opposed to epithelial organs, where we expect cancer if we see this.
They can also have mitosis and vascular invasion and can even be found outside of adrenal (extra-adrenal paragangliomas).
You really have to prove metastasis to prove it’s malignant. In real life we try to apply several features including invasion of other organs, of vessels, if too many mitosis we may comment that the tumor has a higher malignant potential.
MSH
cross reacts with ACTH
causes pigment formation
Addison’s
secondary/tertiary adrenal insufficiency
low ACTH
may be associated with other pituitary defects
i.e. long term steroids, pituitary tumor, genetic, ACTH deficiency
cortisol on GFR
- GFR: Cortisol is also required for normal GFR.
- Excess cortisol à increases the GFR
- When the prof’s dog, India, was on prednisone, she had to be taken out to peeing much more often. The dog also had diabetes insipidus.. So you can imagine.
- Diabetes insipidus is a lack of vasopressin activity, which results in dilute urine. For diagnosis, test for specific gravity of the urine.
- Prof’s dog had a very low urinary specific gravity. The dog was not given water for overnight, but the specific gravity did not change. The dog was then given DDAVP, which raised the specific gravity. This confirmed that the dog lacked vasopressin.
what disorder is ACTH the highest?
ectopic ACTH
ACTH-dependent Cushing’s Syndrome
High ACTH
- Cushing’s Disease (pituitary tumor) - most common
- ectomic ACTH (carcinoid tumor)
Rare: ectopic CRH secretion
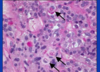
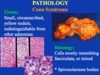
cromaffin cells
in adrenal medulla
neuroendocrine granules with epinephrine and NE
granular appearance

primary glucocorticoid deficiency
Addison’s disease - destricution of the adrenal (infection, autoimmunity, trauma)
high ACTH - can cause darkening of the skin due to crossreaction with receptors for MSH (skin pigment formation)
melanogenic agent
ACTH
associated with hyperpigmentation
hypercortisolism
cushing’s syndrome - exogenous or endogenous
cushing disease - pituitary adenoma - high ACTH
adrenal cushing syndrome - hyperplasia/adenoma of adrenal - independent of ACTH
ectopic cushing - neoplasm producing ectopic ACTH
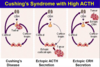
On the left, there’s elevated ACTH, either because of pituitary tumor or ectopic paraneoplastic production (such as tumors in lungs or other neuroendocrine tumors). The high ACTH works bilaterally, so the adrenals are hyperplastic and enlarged.
IF exogenous cause (meds), these are gonna suppress ACTH which means that adrenals are atrophic! These patients we don’t stop cortisol suddenly because the cortex won’t function properly without transition.
IF we have tumor in adrenal that produces cortisol, it too will suppress ACTH which will also cause bilateral atrophy of cortex.
Some causes are caused by idiopathic hyperplasia where there’s suppressed ACTH but maybe something affects the ACTH receptors and stimulates them.

cortisol binding protein
- Glucocorticoids are secreted; they are ~90% bound to a serum-binding protein—cortisol binding globulins (CBG).
- CBG is similar to TBG in terms of its physiology and biochemistry.
- It is the free form of cortisol that is active.
- CBG-bound forms are inactive.
- Like TBG, CBG levels can change depending on factors such as the liver disease.
- Free cortisol is able to diffuse into the cell, bind to a receptor within the cytosol (usually paired w/ heat shock protein or chaperone protein, which can dissociate after receptor-hormone binding).
long term effect of ACTH
increase size and complexity of organelles
increase size and number of cells
via cAMP

congenital adrenal hyperplasia
Spectrum of inherited metabolic errors affecting one of the enzymes involved in the biosynthesis of cortical steroids

all corticosteroids come from precursor cholesterol then you get aldosterone, cortisol, testosterone, etc.
There are many enzymes involved in these steps [o rly]. In congenital adrenal hyperplasia, more than 90% of cases are from 21hydroxylase deficiency - no cortisol and channel to make more androgens!
bilat hyperplastic adrenal glands (10-15x normal), thickened nodular cortex, no negative feedback
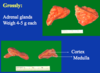
layers of the adrenal cortex
out to in:
glmerulosa
fasiculata
reticularis
- The outer cortex
- Comprises of about 80-90% of the gland
- Made of cells that are derived from the mesoderm

cholesterol desmolase
side chain cleavage enzyme
stimulated by ACTH
cleaves cholesterol to prenenolone

cortisol on CNS
- CNS: Glucocorticoids modulate emotional tone and wakefulness.
- Excess cortisol can cause frank psychosis, euphoria, and/or depression.
- If you think someone has psychiatric illness, you not only have to check their thyroid status but also find out if they have Cushing’s Disease or if they are on steroid for some other reason.
- Answer to a student question (can’t hear the question): “Both. There’s a basal level that’s acting all the time. By the permissive effect, you know, a lot of these things, you don’t see what’s happening. You only see what’s happening, especially with cortisol, if you have too much or not enough. So either case, you see CNS effects. What that tells you is that a normal level was required for just normal CNS functioning.”
pheochromocytoma
uncommon tumor of chromaffin cells that produces catecholamines
hypertension (intermittent/paroxysmal) or sustained
symp: headaches, palpitation, sweating
labs: high urinary catecholamines and metabolites
This is a fascinating tumor that arises on account on chromaffin cells. It was known as the 10% tumor to make it easier to memorize.
10% occur extra-adrenally (the extra-adrenal paragangliomas) and supposedly have higher risk of malignancy, so 90% occur in the medulla.
10% of those in medulla are malignant.
10% are familial [reads point] so 90% are sporadic. Nowadays genetic testing shows that genetic mutations can be seen also in pts that don’t’ seem to be in families with these conditions, and that’s why this 10% number doesn’t apply.
The final 10% are bilateral, and there’s a higher likelihood if you’re dealing with familiar.
congenital adrenal hyperplasia
•21-hydroxylase deficiency – That’s number 3 here (on the diagram).
- If you have a mutation that causes this enzyme activity to be decreased, or to be lacking altogether, what will happen? à The precursors will all back up.
- You will get excess 17alpha-hydroxyprogesterone and excess 17alpha-hydroxypregnenolone.
- They will come down the androgen pathway.
•You get decreased cortisol, decreased aldosterone, and increased androgens.
- In children born with this defect, you can have sexual identity problems.
- Females born with this can have fused labia, enlarged clitoris. They can be misidentified as male.
- There are also severe metabolic problems depending on how severe the 21-hydroxylase deficiency is.
- Lacking cortisone and aldosterone can potentially be fatal.
- Again, what you can see as a side effect is an increase in production of androgen precursors and a resulting increase in circulating androgens. Androgen precursors are converted peripherally to testosterone and dihydrotestosterones.

adrenal gland:

infectious causes of addison’s (chronic insufficiency)
granulomas replace cortex and medulla

primary adrenal insufficiency
high ACTH
i.e. Addison’s wipe out entire adrenal gland
What are the effects of epinephrine?
The only thing you have to remember about all this is that everything makes sense except the effect of glucocorticoids on glycogen. For some reason, it stimulates storage of glycogen rather than mobilization
- Epi then has all of the counter-regulatory effects on fuel metabolism that the glucocorticoids have.
- With the exception that instead of increasing glycogen synthesis, it decreases glycogen synthesis and increases glycogenolysis.
- In adipose tissue, the effects are the same. They increase the fatty acid mobilization.
- There are also other effects: adrenergic effects that facilitate fight-or-flight.
- Vasodilation of certain blood vessels to the skeletal muscles
- If you have to run away, you want to have oxygenated blood to your muscles.
- Increase of HR, contractility, and CO
- Constriction of peripheral blood vessels
- You don’t need peripheral circulation. You need the blood elsewhere.
- For example, you don’t need blood to the internal organs when you have to run. No need to take bathroom breaks if you are in trouble and need to get away.

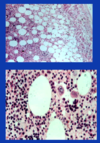
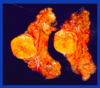
myelolipoma
There are many other tumors that affect the adrenal gland that are similar to tumors that occur elsewhere in the body. The one I want to mention because it has distinct features is myelolipoma [reads slide]. It looks fatty because it has fat mixed in with hematopoetic cells and megakaryocytes.
catecholamine synthesis
tyrosine (like thyroid) –> dihydroxiphenylalanine –> dopamine –> NE –> Epi

- Tyrosine is the precursor for catecholamines.
- Tyrosine was also the precursor for the thyroid hormones. Tyrosine is an important amino acid.
- The first step in conversion of Tyrosine to epinephrine is the tyrosine hydroxylase step.
- This step is stimulated by stress response. When the pre-ganglionic sympathetic fiber fires, the acetylcholine is released. Ach then opens up the sodium channels and causes a depolarization. You’ll have a typical stimulus-secretion coupling, such that epinephrine is released from these cells.
- The epinephrine is stored in granules.
- Tyrosine hydroxylase is subject to feedback inhibition.
- When epinephrine levels are high, it is inhibited.
- If you stimulate secretion of epinephrine, the intracellular levels will drop, and this enzyme will be activated.
- You get formation of dihydroxyphenylalanine and then dopamine.
- Dopamine is converted to norepinephrine.
- Norpepinephrine is then converted via phenylethanolamine N-methyltransferase to epinephrine.
- Phenylethanolamine N-methyltransferase is under the control of glucocorticoids.
primary hyperaldosteronism
10% of hypertensive patients
autonomous production of aldosterone with suppression of plasma renin levels
35% - aldo-producing adenomas (conn syndrome) - surgically correctable HTN
60% - bilat idiopathic hyperplasia, unknown stimulant (medically treated HTN)
Rare: familial, carcinoma
How do you prove that a patient truly has Cushing’s syndrome (not pseudo Cushings)?
blood/salivary cortosil at midnight
OR
24h urine free cortisol
-First thing to do is distinguish real Cushing’s from pseudo-Cushing’s. Stressful states can produce symptoms that look like Cushing’s. It can also be depression itself or alcoholism that can you have pseudo-Cushing’s. You can look Cushinoid but you really don’t have Cushing’s.
what is the most common cause of cushing’s syndrome?
medications
embryonic origin of the adrenal medulla
ectoderm (neural crest)
makes catecholamines

Dexamethasone Suppression Test
screening for Cushing’s
low: give 1 mg dexamethasone at midnight and test 8a cortisol
should suppress cortisol

discriminatory symptoms of cushing’s
hypertension
diabetes
osteoporosis and fractures

Treatment of Cushing’s Disease
- transsphenoidal surgery (80% cured)
- radiation to pituitary
- Decrease pituitary ACTH release (dopaminergic agonists, somatostatin receptor blockers)
- adrenal destruction (surgical or meds)
what does the zona reticularis make?
•Glucocorticoids are generally thought to be made by the zona fasciculata, while androgens are thought to be made by zona reticularis, although there now seems to be overlap between these two zones.

immediate effects of ACTH
activate enzymes and gene transcription through cAMP

adrenal virilism
disorders of sexual differentiation caused by increased adrenal production of androgens
androgen-secreting adrenocortical neoplasm
congenital adrenal hyperplasia (CAH) - autosomal recessive, inhereited metabolic errors affecting one of the enzymes in the biosynthesis of cortical steroids