Acids and Bases Flashcards
What does the pH scale measure?
(level 3 )
The acidity or alkalinity of a substance
What is the number range of the pH scale?
0-14

What is the pH range for acids?
Less than 7
(do NOT say 1-6)

What is the pH range for alkalis?
Greater than 7

(do NOT say 8-14)
What is a pH “indicator”?
A substance that changes colour in the presence of an acid or alkali.
What is the pH value for neutral solutions?
7
Name 3 indicators used in the lab
pH paper
universal indicator
red cabbage indicator
What does pH measure?
(level 4)
The concentration of H+ (aq) ions in a solution
What is the acidic ion?
The H+ (aq) ion
Definition of an acid
A solution with a higher concentration of hydrogen (H+) ions than hydroxide (OH-) ions

How is concentration of hydrogen ions represented?
By using square brackets
[H+ (aq)]
What effect does diluting an acid have on its pH value?
It increases it towards 7

What factor of dilution will increase the pH value of an acid by 1?
A dilution factor of 10
( adding 90ml of water to 10 ml of acid to make 100 ml for example)
What is the alkali ion?
The OH- (aq) ion
What makes solutions alkaline?
A solution with higher concentration of hydroxide ions than hydrogen ions

What effect does dilution of alkalis have on their pH value?
It decreases it towards 7

What factor of dilution will decrease the pH value of an alkali by 1?
A dilution factor of 10
( adding 90ml of water to 10 ml of alkali to make 100 ml for example)
Why do neutral solutions a have pH of 7?
Because they have equal concentrations of hydroxide ions and hydrogen ions
How is concentration of hydroxide ions represented?
By using square brackets
[OH- (aq)]
Why can the concentration of OH- (aq) NEVER be zero even with very low pH values?
Because
[H+ (aq)] x [OH- (aq)] = a number that is NOT zero
What is acid rain?
Rain that falls with a pH below 5.5
What are some uses of acids in food industry
- flavourings
- preservatives
Which 2 gases are mainly responsible for acid rain?
sulphur dioxide
nitrogen oxides

What is an “acidic oxide” ?
A NON-METAL oxide that is soluble in water and lowers the pH below 7.

What is an “basic oxide” ?
A METAL oxide that is soluble in water and increases the pH above 7
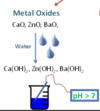
What are the 3 common lab acids?
Hydrochloric acid
Sulphuric acid
Nitric acid
What is the molecular formula and the ionic solution formula for hydrochloric acid?

What is the molecular formula and the ionic solution formula for sulphuric acid?

What is the molecular formula and the ionic solution formula for nitric acid?

What are the 3 most common lab alkalis?
Sodium hydroxide
Barium hydroxide
Potassium hydroxide
What is the ionic solid and ionic solution formula for barium hydroxide?

What is the ionic solid and ionic solution formula for potassium hydroxide?

What is the ionic solid and ionic solution formula for sodium hydroxide?



