8 - Kidney Disease Flashcards
What is the leading cause of ESRD in most western societies? What is the risk related to?
Diabetic nephropathy - can occur in both type 1 and type II
- Risk related to the duration of disease.
- Risk is multifactorial - 30-40% of pts will develope neuropathy
What is the pathogenesis of hyperfiltration seen in diabetic nephropathy?
- Hyperfiltration commonly in early diabetics.
- Increased GFR due to glucose-dependent afferent arteriolar dilation.
- AngII mediated constriction of the efferent arteriole; angII also causes hypertrophic PT growth.
- Hyperfiltration increases colloid osmotic pressurein the post-glomerular capillaries increasing Na reabsorption in the PT.
Basically, glucose causes afferent dilation and efferent constriction to increase glomerular filtration. This process causes leakage of proteins.
These can be corrected with proper glycemic control.
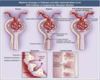
When is renal hypertrophy seen in diabetic nephropathy?
Seen early in onset; size of the kidney may increase by several cms.
Associated with an increase in the number of mesangial cells and capillary loops; increasing filtration SA.
When mesangial changes are seen in diabetic nephropathy?
- Mesangial expansion mediated by glocuse
- Nodular diabetic glomerulosclerosis (Acellular Kimmelstiel-Wilson lesion)
Early mesangial lesions characterized by increase in mesangial cell number and size and increased deposition of ECM.

What is the pathogenesis of hte proteinuria seen in diabetic nephropathy?
Widening of the glomerular basement membrane causes accumulation of type IV collagen and net reduction in negatively charged heparin sulfate.
Podocyte changes.
Serum proteins cross the BM due to the disrupted texture, gaps, and holes.

What is the pathogenesis of the fibrosis seen in diabetic nephropathy?
Tubulointerstitial fibrosis seen in early DN cauesd by release of growth factors - correlates with prognosis (ie more fibrosis = bad)
Tubular cells change and become fibroblasts.
High glucose concentration and advanced glycation end products (AGEs) further stimulates this process.
Describe the stages of diabetic nephropathy and what this tells us about our ability to diagnosis?
Within the first 5 years you have increased GFR but no albumineria.
Eventually (at about year 10-15) GFR decreases and proteinuria increases.
This is why you can’t diagnose diabetic nephropathy for until 15 years.

What is seen in stages 1 and 2 of diabetic nephropathy?
Stage 1: onset of diabetes; increased GFR from hyperfiltration, glomerular hypertrophy on biopsy, reversible and transient proteinuria
Stage 2: clinically asymptomatic but biopsy shows mesangial expansion and GBM thickening
What is seen in stages 3 and 4 of diabetic nephropathy?
Stage 3: early nephropathy, development of HTN, persistant microalbumineria
Stage 4: overt proteinuria, GFR starts to decline; 50% will reach ESRD within 7-10 years. Retinopathy in 90-95% of pts.
What is seen in stage 5 of diabetic nephropathy?
End-stage renal disease; renal replacement therapy needed. Occurs in avg ~15 years after onset of TIDM in pts who develope proteinuria
What are co-morbidities of diabetes mellitus?
- HTN
- Neuropathy
- Vascular changes
- Increased mortality
What are complications of diabetic nephropathy?
- Diabetic retinopathy
- Polyneuropathy
- Macrovascular complications (5x more frequent): stroke, coronary heart disease, peripheral vascular disease.
What is mortality from diabetic nephropathy related to?
The degree of proteinuria - more protein in the urine is correlated to worse mortality.
What is the treatment for diabetic nephropathy?
- HTN treatment
- Glocuse control
- Reduction of proteinuria
- Lipid lowering therapy
- Lifestyle modification
What did the diabetes control and cmoplications trial (DCCT) tell us about treating diabetic nephropathy?
Good glycemic control helps decrease the risk of progression.
- reduction in progression from normoalbumineria to microalbuminuria
- decrease in microvascular complications, specifically retinopathy
- decrease in CV sequelae, which persisted despite later deterioration in glycemic control
What medications can be used to reduce proteinuria seen in diabetic nephropathy?
RAA system blockade
- renoprotective independent of BP
- reduces proteinuria
- may cause up to 30% decline in GFR but renoprotective in the long term
- works through renal hemodynamic changes and blocking non-hemodynamic effects of AngII
What is the lipid panal of most patients with diabetic nephropathy? What does this tell us about what medicatons can be used to treat them?
- Low HDL
- High TGs
- Smaller LDL particle
In type 2 diabetes with diabetic nephropathy, treatment with statins provides substantial cardiovascular benefit.
What are two non-diabetic nephrotic syndromes?
Amyloidosis and light chain deposition disease
What is amyloidosis? What happens when it affects the kidneys?
Characterized by deposition in extracelluilar spaces of a proteinaceous material.
In the kidneys: light chains secreted by a single clone of B cells are deposited.
- usually lambda light chains (AL)
- 20% of cases associated with multiple myeloma
What symptoms can result from amyloidosis in the kidneys?
Often enlarged and HTN is absent even when renal function is impaired.
Proteinuria, mainly albumin, occurs in the absence of microscopic hematuria.
Tubular defects from amyloid deposits cause renal tubular acidosis and polyuria-polydipsia (alot of urine and increased thirst).
What is seen ion immunofluorescence in renal amyloidosis?
Lambda light chains

What are extra-renal manifestations seen in renal amyloidosis?
AL amyloidosis may infiltrate almost any organ other than the brain:
- Restrictive cardiomyopathy in 1/3
- GI motility disturbances
- Splenomegaly
- Peropheral nerve: sensory polyneuropathy
- Purpura: around eyes, face and upper trunk
- Joint - shoulder pain/swelling
- Macroglossa (large tongue)

What is light chain deposition disease? What do pts develop?
Deposition of excesss immunoglobulin light chains in the kidney: usually Kappa chains.
50% coexist with multiple myeloma
Pts develop:
- proteinuria
- hematuria
- chronic renal insufficiency
What do you see on light microscopy, immunofluorescence, and electron microscopy in light chain deposition disease?
LM: nodular glomerulosclerosis
IF: positive for kappa staining
EM: granular deposits along GBM

What are two hereditary glomerular diseases?
Alport syndrome and thin basement mambrane
What causes alport syndrome and who gets it typically?
Typically males.
Most commonly X-linked recessive but can be aut recessive.
Mutation of the COL4A5 gene on chromosome Xq22 which encodes alpha5 chain of type IV collagen causing defect in the basement membrane.
What are the renal manifestations in alport syndrome?
Hematuria
Proteinuria absent early but develops eventually in all males with X-linked alport syndrome (XLAS) and in both males and females with aut. recessive alport syndrome.
HTN
ESRD in all affected males with XLAS (90% by age 40)
What are extra-renal manifestations of alport syndrome?
Cochlear defects
Ocular defects
- This is because type IV collagen is found in the kidneys, eyes, and ears
Leiomyomatosis (less common)
What is seen on light microscopy and electron microscopy in alport syndrome?
LM: early in diesase: glomeruli may appear normal. Later, global and segmental glomerulosclerosis, interstitial fibrosis.
EM: variable thickening, thinning, basket weaving, and lamellation of the glomerular basement membrane.

What is the treatment for Alport’s disease?
Non disease-specific therapy - RAAS blockade
Renal replacement is eventually necessary
Transplant: 2-3% will get anti-GMB disease
What causes thin basement membrane disease (benign familial hematuria)?
Usually aut. dominant inheritance.
Continuous or intermittent microhematuria with or without gross hematuria.
Previously considered benign: proteinuria, HTN, and ESRD are unusual.
Extra-renal features are RARE.
What is seen on light microscopy, immunofluorescence, and electron microscopy in thin basement membrane disease?
LN: normal glomeruli
IF: negative
EML thin glomerular basement membrane (usualy less than 200 nm)

What is the treatment for thin basement membrane disease?
Reassurance
Should be followed, but very small real risk of progression to ESRD.
Basic metabolic panel (BMP), urinalysis, and BP monitered every 1-2 years.


