5. Signalling Mechanisms in Growth and Division Flashcards
(28 cards)
What happens to cells in the absence of growth signals?
- They go into G0 (quiescent phase)
- E.g. hepatocytes are usually in this phase as they don’t divide very regularly
What is the link between c-Myc and cell-cycle entry?
- The concentration of Myc is really low when the cell is in the quiescent G0 phase
- If you trigger cell division (e.g. by adding a growth factor) you get a rapid and dramatic rise in Myc, which then plateaus at an intermediate level
- This correlates with cells moving out of G0 and into G1
- Myc is a transcription factor - it is a protein that controls the expression of other genes
- In the case of Myc, many of the genes it controls are involved in the cell cycle (hence why it is elevated when the cell wants to enter the cell cycle)

Name the key components of signalling pathways.
- Regulation of enzyme activity by protein phosphorylation (kinases)
- Adapter proteins
- Regulation by GTP-binding proteins
Describe the stimulation of growth factor by signalling pathways
Explain growth factor stimulation by signalling proteins
- Growth factor (e.g. hepatocyte growth factor) binds to a receptor (usually a tyrosine kinase type receptor)
- It then activates a small GTP-binding protein (Ras)
- This then triggers a kinase cascade
- The early stage of cell cycle triggering is very fast - this then triggers the activation of genes that are required for the progression of cells throught the cell cycle - this is slower because it requires transcription and translation to take place (NOTE: it takes an hour or so to induce a gene that is required for progression through the cell cycle.)
- One of the genes triggered early in the cascade is c-Myc, which then goes on to regulate the expression of many other genese
Describe the binding of the peptide growth factor to the receptor.
The receptors normally sit on the plasma membrane as monomers but most growth factors are dimeric
- When the dimeric growth factor binds to two receptor tyrosine kinase molecules, it brings them closer together
- When the receptors are close together, the tyrosine kinase domain is able to cross-phosphorylate the partner receptor (you get multiple cross-phosphorylation of several tyrosine residues)
- Tyrosine kinases use the gamma phosphate of ATP to phosphorylate tyrosine residues in proteins
- The phosphorylated domains on the tyrosine kinase receptors act as docking sites for adaptor proteins (which are recruited to the activated tyrosine kinase receptors)
- The adaptor proteins contribute to downstream signalling.
This is one of the first places that you can interfere with growth factor signalling.
- EXAMPLE: there is an antibody called herceptin that inhibits the her2 receptor tyrosine kinase - this is important in a number of tumours e.g. breast cancer
- The anti-Her2 antibody can be used to block the early stage of growth stimulation
- One of the important adaptor molecules that is recruited is called Grb2

Explain the need of adaptor proteins.
- Adaptor proteins are often modular- there are different domains that are mixed and matched to give the protein different properties
- These different domains are important in molecular recognition
- The adaptor molecules have no enzymatic function - they don’t do anything other than bringing other proteins together
- An extremely important adaptor molecule in growth factor signalling is Grb2
-
Grb2 only has TWO types of protein-protein interactions:
- SH2- binds to the phosphorylated tyrosines of the receptor
- SH3 (there are two copies) - bind to the proline rich regions of other proteins

What happens to Ras after the peptide growth factor has bound to the receptor?
- At this point Grb (adaptor protein) is bound to the receptor protein tyrosine kinase via its SH2 domain and it binds to a protein called Sos through the SH3 domains
- Sos is an exchange factor for Ras (a signalling molecyle that sits in the membrane of the cell)
- NOTE: Grb is always bound to Sos
- When Sos is close enough to Ras is can activate it
- Sos allows the exchange GDP for GTP in Ras to form a GTP bound form of Ras
- This changes the conformation of Ras, which puts it into the active state that can signal downstream and can allow the propagation of the signal

If you can interfere with the membrane binding of Ras, you can make a good anti-cancer therapy
Explain the molecular switch in GTP-binding proteins (e.g. Ras)
- Ras is a GTP binding protein and they are very powerful molecular switches
- They are either on or off:
- On-GTP bound
- Off - GDP bound
- Under the influence of appropriate signals, the GTP can replace the GDP to make Ras active
- NOTE: This is NOT phosphorylation - it is merely the exchange of GDP for GTP (catalysed by Sos)
- This is a self-regulating system so Ras can turn itself odd
- It is able to hydrolyse GTP to GDP to turn itself off - there is an intrinsic GTP hydrolysis capability
- The hydrolysis itself can be stimulated by another family of proteins - GTPase activating proteins (GAPs)
- So the cycle of GTP binding proteins is almost always controlled by:
- Exchange factors (e.g. Sos) that turn it ON
- GTPase activating proteins (GAPs) that turn it OFF
- In cancer, you find that the Ras protein is mutated in ways that cause the Ras protein to constantly be in the GTP bound form

Describe potential mutations to Ras.
-
V12Ras
- The glycine residue in position 12 of the Ras protein has changed to VALINE due to Ras gene mutation
- The side chain goes from being a simple hydrogen (in glycine) to a hydrophobic side chain
- This prevents GAPs from binding to Ras, thus meaning that Ras can’t turn off very easily.
-
L61Ras
- Glutamine in position 61 is converted to LEUCINE
- This is a single base change in the genome
- The side chain goes from being an amide to a hydrophobic side chain
- This mutation inhibits the intrinsic GTPase activity of the Ras protein
- Ras ends up constantly being in the GTP bound state and therefore giving growth stimulatory signals

What does Ras do?
- GTP bound Ras binds to a kinase and then that kinase activates several other kinases
- The top kinase phosphorylates the next kinase, then that kinase phosphorylates the next kinase and so on…
- The kinase cascade that is involved in the growth stimulatory signalling is known as the ERK cascade (extracellular signal-regulated kinase cascade)
- ERK is a specific example of this type of cascade - specific to growth stimulatory signalling
- The family of these kinase cascades are called the MAPK cascades (mitogen-activated protein kinase cascade)
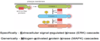
Describe the Extracellular signal-regulated kinase cascade.
- B-Raf is an oncogene – mutationally activated in melanomas
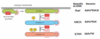
What happens at the end of the ERK cascade?
- At the end of the cascade, the last kinase phosphorylates a number of proteins and changes their activity
- Among the proteins that are phosphorylated are gene regulatory proteins(transcription factors)
- Once phosphorylated, the transcription factors go on to regulate gene expression
- One of the most importnat genes that is turned on is the c-Myc gene
- So, activating the growth factor pathway through the kinase cascade leads to the activation of a gene regulatory protein, which stimulate c-Myc production
- Myc and Ras are key molecules in stimulating growth - they are commonly found to be mutated or over-expressed in many human tumours
- Myc and Ras are ONCOGENES
Explain the action of cyclin-dependent kinases (Cdks).
- These are serine-threonine kinases (NOT tyrosine kinase)
- These Cdks are in the cell throughout the cell cycle but they are not activated until they bind to an activating protein called a cyclin
- The Cdks are ALSO controlled by phosphorylation - this ia an extra level of control

Explain the role of cyclins.
- Cdks are activated by binding to cyclins
- Cyclins are transiently expressed during the cell cycle
- Once they have activated the Cdks, the cyclins are degraded
- The cyclins are regulated at the level of expression

Name points at which different cyclin-Cdk complexes trigger different events.
- There are different cyclin-Cdk complexes that trigger different events in the cell cycle
- The M-phase promoting factor controls the progression through mitosis - the Cdk is being activated by a mitotic cyclin
- Once the Cdk has fulfilled their role, the cyclin is degraded and the Cdk is turned off
- At the start of DNA replication, there is a Cdk that is turned on by binding to an S phase cyclin
- Then this cyclin is degraded once the Cdk has carried out its function

What does the mitotic promoting factor consist of?
- Cdk1
- Mitotic cyclin (usually cyclin B)
Explain the regulation of Cdks by phosphorylation.
- Cdk1 binds to cyclin B
- This Cdk-cyclin is usually inactive on its own
- It has another level of regulation, which is PHOSPHORYLATION
- There are 2 phosphorylation reactions that regulate Cdk activity
- Cdk has to be activated at specific sites to become activated
- The activation is performed by Cdk activating kinase (CAK)
- Balancing this is an inhibitory kinase called Wee1
- CAK puts activating phosphorylation onto Cdk1
- Wee1 puts and inhibitory phosphorylation onto Cdk1
- Even though Cdk1 is bound to cyclin, it needs the inhibitory phosphate to be taken off before it can function
- Cdc25 takes off the inhibitory phosphate that was put on by Wee1
- Then you get mitotis promoting factor
- Overview of steps in activating MPF:
- Cyclin binding to Cdk
- Activating phosphorylation by CAK
- Removal of the inhibitory phosphate (that was put on by Wee1) by Cdc25

What is active MPF (mitosis promoting factor) able to do to Cdc25?
- Active MPF is able to phosphorylate Cdc25 to increase its activity
- This is a form of positive feedback that drives mitosis
- As soon as you get active Cdk1, then you activate more Cdc25, which in turn, leads to more dephosphorylation of the inhibitory site and this positive feedback pushes the cell through mitosis

Name the different cyclings throughout the cell cycle
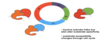
- Different cyclins and Cdks control different stages of mitosis
-
G1/S
- Cdk2
- Cyclin E
-
S
- Cdk2
- Cyclin A
- Important: the same Cdk is being used in G1/S phase and S phase but they are doing different jobs
- This is because when cyclin binds to Cdk it actually changes its substrate specificity so that it can phosphorylate different substrates depending on which cyclins are bound to it
- It also changes substrate accessibility - the substrates available in G1/S will be different to those available in S phase
- This features allows you to use the same Cdk in different stages of the cell cycle.

What does c-Myc stimulate transcription of? What happens after the trancription?
- Growth factors come and bind to the receptor protein tyrosine kinase
- Through Ras, this triggers a kinase cascade
- This leads to the phosphorylation of transcription factors that turns on the expression of c-Myc
- Myc is a transcription factor and one its jobs is to stimulate the transcription of cyclin D
- Cyclin D activates Cdk4 and Cdk6. (which triggers the cell cycle)
- Cdk 4 and Cdk6 stimulate synthesis of cyclin E

Explain the role and peaks of cyclin throughout the cell cycle.

- RECAP: growth factor leads to the production of Myc (a transcription factor), which, in turn, stimulates the synthesis of cyclin D. Cyclin D leads to the activation of Cdk4/6-cyclin D complex
- This Cdk-cyclin complex then stimulates the synthesis of the next cyclin and they then become sequentially active - each cyclin is involved in stimulating the synthesis of the next cyclin
- This gives direction to the cell cycle
- It also gives timing because it takes time for the concentration of the cyclin to build up so the appropriate Cdk is activated

What do MPF and Start kinase phosphorylate?
- MPF phosphorylates proteins involved in mitosis
- E.g. Nuclear lamina - needed for breakdown of nuclear envelope
- Breakdown of the nuclear envelope is caused by phosphorylation of the nuclear lamins
-
Start kinase (from G1 phase to S phase) is a complex of Cdk2 and cyclin E - this phosphorylates substrates needed for that phase
- The most important protein that is phosphorylated by start kinase is RETINOBLASTOMA
- Retinoblastoma is a key protein in regulating the cell cycle - it is present throughout the cell cycle
- In the resting G0 state, retinoblastoma is unphosphorylated
- In this state, it binds to and sequesters a family of transcription factors called E2F
- When E2F are held in the cytoplasm by pRB (unphosphorylated retinoblastoma), everything is turned off
- Retinoblastoma is a target for Cdk4/6-cyclin D kinase (which becomes active following Myc induction)
- It starts to phosphorylate the retinoblastoma protein and as the protein becomes phosphorylated, it loses its affinity for E2F so it releases it
- E2F can then bind to promoters in the nucleus of genes that are involved in cell cycle progression
- One of the targets of the E2F transcription factor is the gene for cyclin E - the next cyclin that is required for cell cycle progression
Retinoblastoma acts as a brake in the cell cycle - when it is unphosphorylated, it sequesters E2F and prevents the gene expression so the cell cycle does not progress - it is a TUMOUR SUPPRESSOR GENE







