11. Invasion Flashcards
(28 cards)
What molecular mechanisms regulate motility?
The molecular mechanisms that regulate motility (in terms of detachment from primary tumours and migration) are :
- MICROFILAMENTS
- CYTOSKELETAL PROTEINS
- SIGNALLING PROTEINS.
- Regulation of ACTIN DYNAMICS is involved in motility.
Explain how tumours metastasise.
- Normally, hyper-proliferation leads to a BULK of cells (solid tumour). The cells still have some contact with each other, and are bound to each other within the tissue.
- As soon as the cells de-differentiate, they break away from the basement membrane. The metastatic tumour cells can invade veins and exit at different sites in the body.
- Once tumour cells exit the venous/lymphatic systems, they can COLONISE and METASTASISE at long distances from the site of origin.

What are the types of tumour cell migration?
- There are different ways in which the cells can migrate. These migratory strategies always exist, but tumour cells majorly exploit them.
- 2 types of cell motility:
- Individual (single cell migration)
- Collective (group of cells)
- Both types of motility require integrins and proteases. Collective migration requies modulation of cell-cell contacts, and communication between cells. (e.g. gap junctions)
- Different tumour types tend to prefer different methods of migration.
- Amoeboid:lymphoma, leukaemia, SCLC
- Mesenchymal (single cells/chains):fibrosarcoma, glioblastoma, anaplastic tumours
- Cluster/cohorts:epithelial cancer, melanoma
- Multicellular strands/sheets:epithelial cancer, vascular tumours

Name the types of
How do tumour cell metastasis mimic morphogenic events.
- Breast feeding.
- When tissue has to grow, cells bud to grow and branch in order to form the mammary glands. The whole tissue will invade its surroundings and grow around.
- Vascular Sprouting
- Whenever vessels need to be remodelled, cells must invade the surrounding areas as a STRUCTURE. They cannot migrate individually.
- If a confluent monolayer is SCRAPED, cells sense spaces between them. Immediately, they will MIGRATE TOGETHER to close the gap. This is how HEALING works, using COLLECTIVE MIGRATION. Tumour cells demonstrate this migration but it is not organised – cells migrate EVERYWHERE. Contact inhibition of migration is INEFFECTIVE in tumour cells.
What stimuli are required by the cells to move?
- Organogenesis and morphogenesis
- Wounding
- Growth factors/chemoattractants
- De-differentiation (tumours)
The cell has protrusions around the periphery. The red is the actin-based cytoskeleton, and the green is the microtubule cytoskeleton. When the cells are stimulated to migrate, they change their morphology. They move in the direction of migration.
There is a directionality (polarity) of movement that is essential. Cells also need to know when to stop – CONTACT-INHIBITION MOTILITY does this. Cells need to engage into specialised structures – these structures vary depending on the type of cell motility (focal adhesion, lamellae, filopodia).

Why is the substratum important in migration of cells?
- If the cells cannot attach, they CANNOT migrate by the standard processes
- Focal adhesions hook onto the ECM matrix, and grab it to provide points where the cells can attach
- Cells attach to the ECM using focal adhesions, and engage their cytoskeleton to connect
- TRACTION FORCES ARE GENERATED
- The hooking is mostly done by dimer integrin receptors
- They are transmembrane protein (one transmembrane domain) with a short cytoplasmic tail.
- The tail has no enzymatic activity
- Integrins just have docking places for cytoskeletal protein
- They come around, to form a plaque/complex of proteins
- The plaque mediates the interaction with actin fibres.

What structures are need for motility?
- FILOPODIA
- finger-like protrusions rich in actin filaments
- Vinculin is an ACTIN-BINDING PROTEIN
- We can think of filopodia as finger-like projections that sense the environment, tell the cell where they should attach.
- They are exploratory structures that the cells use to coordinate their movement.
- LAMELLIPODIA
- Sheet-like protrusion rich in actin filaments
- Lamellipodia is slightly more complex, because it is a sheet of membrane that expands
- The cell migrates in a certain direction, and the sheets of membrane project to the front of the cell (in the same direction)
- The sheets then ruffle back, so that the cell can move.

Why is control needed in cell movement?
- Within a cell, control is needed to coordinate what is happening in different parts
- Control is needed to regulate adhesion/release of cell-extracellular matrix receptors
- From outside to respond to external influences – sensorsand directionality
Define Hapoptatic and chemotactic motility.
- HAPOPTATIC MOTILITY: directional motility or outgrowth of cells with no purpose
- CHEMOTACTIC MOTILITY: movement in response to a chemical stimulus (this is a purposeful response)
- However, these two types of motility still use the same core machinery
- Cell movement = cell changing shape
How do local adhesions aid cell motility?
- The focal adhesions act like feet. They allow the cell to attach and protrude. Cell protrusions extend (lamellipod) and attach again to the ECM. This is the basis of cell movement.
- Once another focal adhesion is made, the back of the cell must contract using energy to move the cell forward.
- The cell moves, one step at a time. Old adhesions are left behind, and the cell is ready to move forward

Describe actin filament polarity.
- Actin is a monomer and is a fundamental molecule in cell. Actin can polymerise in the cells. Actin monomers are polarised – they have different structures on each end.
- There is a complex regulation between the monomer and filamentous states.
- A signal reaches the cell, and is recognised by the cell. The cell moves towards the source –> there is a rapid disassembly of the filaments –> rapid diffusion of monomers of actin –> reassembly at the side of the cell that is going towards the source –> repolarisation of cell.

Describe the filamentous structure of filapodia, stress fibres and lamellipodia.
- In the filapodium, actin is in its filamentous form, in a parallel arrangement. They bundle together to provide structure to the membranous filopodia projection.
- Stress fibres have an anti-parallel organisation of the filaments. This is necessary to make a contraction. During contraction, actin filaments slide along each other and shorten their distance. They contract the whole cell body. They end at the focal adhesions.
- Lamellipodia have no direct fibres, but there are branched and cross-linked fibres (like a net) that provide support to the big sheet of membrane.

Describe nucleation of filaments.
- The nucleation step is the rate-limiting step in the organisation of the cytoskeleton.
- It requires a lot of energy. There are specific proteins that help to form filaments.
- Arp = actin-related proteins. They have similar structures to actin, but they are NOT actin. They can help monomers to form a trimer. Once this step happens, filaments can form.
- The Arp-2,3 complex binds to the minus end of the actin filament to form the initial trimer, and extend the filament
- Limiting step in actin dynamics – formation of trimers to initiate polymerization

Describe elongation of filaments.
- After trimer formation, elongation must occur (extension of the filament).
- Different classes of proteins assists the process. For example, profilin is a protein that binds to G-actin (monomeric actin), and d_rags it over to the actin filament_.
- Thymosin protein binds to actin monomers, but they DON’T bring actin monomers to the filament like profilin does. They more or less inhibit the polymerisation process (act like a brake).

Describe capping of filaments.
- Capping proteins regulate the elongation process of the filament. The capping protein binds the end of the filament and prevent monomers from being added on.
- The filaments are very dynamic. Once adding is blocked, there is a disassembly process that results that –> shortening of the filament.

Describe severing of the filaments.
- The filament size can be regulated by severing. The unsevered actin filament grows and shrinks, adding and removing monomers.
- Severing proteins chop the filament up, which counter-intuitively generates more ends so that filaments can grow more rapidly.
- Gelsolin has two functions – it is a capping and severing protein. The function of gelsolin at any one time resides with its regulation (e.g. modification).

Describe cross-linking and bundling of protein making up filaments.
- Filaments can take different shapes. Cross-linking and bundling proteins do this.
- Fascin will bind filaments together at a particular distance.
- Fimbrin will also bind filaments together, but between those at a long distance from one another.
- Alpha-actinin is a dimer, which binds filaments.
- Spectrin, filamin and dystrophin will cross-link the filaments in particular angles. Each particular protein will make a specific angle with the filaments.
- PROTEINS INVOLVED: alpha-actinin, fimbrin, filamin, spectrin, villin, vinculin.
- Vinculin is a bundling protein.

What is the consequence of filaments being too close to each other?
If the filaments are too close to each other, motor proteins will not be able to come in. Motor proteins include myosin – this comes in to promote sliding, enabling the cell and filaments to contract.
How does branching take place?
- Cross-linking proteins are important, but in the lamellar, they have a very precise angle in the filaments (70 degree angles).
- The Arp-2 complex is the protein responsible for the branching appearance of the filaments as the cells move forward in the lamellar.
- The Arp-2 complex can nucleate(forming a trimer of G-actin) AND branch– here, they bind to the sides of the filament at 70 degrees. This allows nucleation to occur and filaments to elongate outwards.

What must happen to the rigid cell cortex in order for movement?
- When the cell needs to move and project, the rigid cell cortex must be broken. This will allow the cell membrane to flow forward.
- This is called gel-sol transition. Gel is a rigid structure of the actin cytoskeleton. Here, cross-linking proteins are holding the filaments as a mesh.
- If the membrane pushes through, this gel mesh must be broken down. Severing does this. The actin cross-linking proteins are still present, but the filaments aren’t forming a mesh anymore. This allows a sol that can flow. The cytoplasm can move to another area.

What can dysregulation of the actin cytoskeleton cause?
- High blood pressure
- Wiskott-Aldrich Syndrome – WAS (immunodeficiency, eczema, autoimmunity)
- Duchenne Muscular Dystrophy (muscle wasting)
- Bullous Pemphigoid (autoimmune disease)
Explain the participation of different actin activities during cell movement
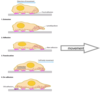
- Proteins can be integrated in the process of directional motility. When lamellipodium extension takes place, there is a lot of actin polymerisation in the lamellipodium.
- In the focal adhesion formation, there is assembling, nucleation, elongation, capping, severing, branching and bundling. All of this occurs together in order to regulate the ways in which the cells protrude and form adhesions.
- At the membrane, there is the gel-sol transition on the cortex. Finally, cells need to contract at the back; otherwise they will be RIPPED APART.
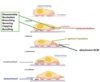
Explain what needs to happen during lamellipod protrusion.
- When the lamellipod protrudes, the membrane protrudes forwards. There is an assembly of filaments. There is branching and capping.
- At the back of the lamella, there is SEVERING, so that you can release the assembly of the filament (there is disassembly). This allows the G-monomers to move to the point in the cell (at the front), where they are needed to make new assemblies.
- There must be a lot of coordination between these activities. The net result is new assembly of actin at the leading edge (provided by monomers at the back of the cell, that have been generated to move the cell).






