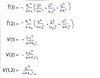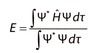4b: Quantum Mechanics Flashcards
(36 cards)
What is the schrodinger equation for a particle in one and three dimensions?

Define the normalisation constant and give its mathematical definition.
The normalisation constant makes sure the vaule of ψ2 is constant over all values.
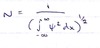
What are the three requirements for ψ?
Single valued at all points
Never infinite
Continuous and smooth (and its derivitive)
Integration over all values must be finite
What is Heisenbergs uncertainly principle?
It is impossible to specify simaltaneously, with arbitrary precision, both the momentum and position of a particle.
ΔpΔx >= ½ħ
What are the limits of the particle in a box?
The potential energy, Vψ, within the box is 0, and is infinite outside the box walls.
What does the energy of a particle in a box depend on?
The mass of the particle and the length of the box.
When can particles in a box tunnel?
When the walls are a finite barrier and the potential of the walls aren’t infinite, there is always a chance of tunneling.
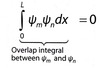
How do you define orthogonal wavefunctions?
How does the number of energy levels change when the particle in a box is expanded to three dimensions?
There is an energy level for each dimension, hence when the energy levels are n= 1, 2, 3; 6 different energy levels with the same energy are possible.
How is the particle in a ring equation different to the normal equation?
The particle in a ring uses moment of inertia because instead of a distance in x, and angle is used to define its position. The boundary condition is therefore that adding 2π doesn’t change the wavefunctions value.
How is a complex wavefunction accounted for?
ψ2 is calculated by ψψ*, each energy level has 2 degenerate energy levels with a positive and negative value of ml.
How is the schrodinger equation different for a harmonic oscillator?
The potential energy is defined by the potential energy of a spring and the force constant, V = ½kx2
The vibrational energy here can never be zero. Reduced mass is used in the case of a diatomic to simplify the equation to a single particle oscillating about a centre of mass.
What is the energy operator called and what is the general operator for other variables.
The energy operator is the hamiltonian operator.
All observables are defined by their own operator, generally called a hermitian operator and they must all give real eigenvalues.
How do you convert from classical variables to quantum?
All observable variables become operators.
How do you adjust the schrodinger equation for time dependance?
As time is another dimension, the wavefunction has to include that variable. This means that energy is no longer directly included in the equation.
What is the equation for the hydrogenic atom and what assumptions have been made?
Assume nucleus is fixed due to large mass ratio difference. The potential energy is defined by Coulombs potential energy.
To account for the nucleus moving, replace the mass of the electron with a reduced mass.

How can the schrodinger equation for the hydrogenic atom be made easier to solve?
By switching from Cartesian (x,y,z) coordinates to spherical polar coordinates (r = radial distance, θ = zenith, φ = azimuth)
This is known as the seperation of variables.
ψ(r,θ,φ) = R(r) Y(θ,φ) = R(r) Θ(θ) Φ(φ)
Where R(r) is the radial wavefunction and Y(θ,φ) is the spherical harmonics/angular components.
Define which quantum numbers the seperated variables of the hydrogenic atom depend on.
R(r) depends on
- Principle quantum number, n = 1, 2, 3…
- Orbital angular momentum quantum number, l = 0, 1, 2, …, n - 1
Θ(θ) depends on
- Orbital angular momentum quantum number, l = 0, 1, 2, …, n - 1
- Magnetic quatum number (magnitude), ml = l, l - 1, l - 2, …, -l
Φ(φ) depends on
- Magnetic quatum number, ml = l, l - 1, l - 2, …, -l
What is the definition of an atomic orbital, a shell and a subshell?
An atomic orbital is a one-electron wavefuntion for an atom.
A shell is all AOs that have the same value of n.
A subshell are AOs with the same value of n but different values of l.
What is the radial distribution function?
r2R(r)2dr which gives the probablility of finding an electron in a shell of thickness dr. The probability across all values of r is normalised to 1.
What values of mL corrospond to pz, py and px?
mL = 0 corrosponds to pz.
mL = ±1 has components in both py and px. Φ is complex for both py and px.
How can real d orbitals be obtained from the different complex values of p-1,-2,1,2?
How can the angular components of both d and p orbitals be simplified?
d0 is real and corrosponds to dz2.
By combining complex orbitals, real orbitals can be found.
By writing them in cartesian coordinates.
How is electron spin defined? How is this applied to a spacial orbital?
The spin quantum number, s = ½
The spin magnetic quantum number, ms = ±½ where the α electron = ½ and the β electron = -½.
The spacial orbital is split from ψ(x,y,z) to ψ(x,y,z)α(ω) and ψ(x,y,z)β(ω).
In simple terms, note all the interactions for a single particle and for two particles, then define each term for the equation with two particles.
Single particle: E = T(1) + V(1)
Two particles: E = T(1) + T(2) + V(1) + V(2) + V(1,2)