3.2.5 Transition Metals Flashcards
Which block are transition metals found in?
d-block
What is a transition metal?
A metal that can form one or more stable ions with a partially filled d sub-level
(d-orbital can contain up to 10 electrons)
State which period 4 d-block elements are transition metals
All period 4 d-block elements are transition metals expect scandium and zinc
What causes transition metals to have special chemical properties?
Incomplete d sub-level
Explain why scandium isn’t a transition metal
- Scandium only forms one ion, Sc3+, which has empty d sub-level
- Sc = [Ar] 3d1 4s2
- When loses 3 electrons to form Sc3+
- Ends up with electron configuration [Ar]
Explain why zinc isn’t a transition metal
- Zinc only forms one ion, Zn2+, which has full d sub-level
- Zn = [Ar] 3d10 4s2
- Forms Zn2+ = loses 2 electrons both from 4s sub-level
- ∴ keeps full 3d sub-level
Transition metals form ______ ions
positive
s electrons removed first & then d electrons


Name 4 special chemical properties of transition metals
- Form complex ions
- Form coloured ions
- Good catalysts
- Exists in variable oxidation states
Why do elements show variable oxidation states?
- ∵ energy levels of 4s and 3d sub-levels are very close to one another
- ∴ different no. of electrons can be gained or lost using fairly similar amounts of energy
Oxidation state +7
State colour of MnO4-
Purple
Oxidation state +6
State colour of Cr2O72-
orange
Oxidation state +5
State colour of VO2+
yellow
Oxidation state +4
State colour of VO2+
blue
Oxidation state +3
State colour of V3+
green
Oxidation state +3
State colour of Cr3+
violet/green

Oxidation state +3
State colour of Fe3+
purple/yellow

Oxidation state +2
State colour of V2+
Violet
Oxidation state +2
State colour of Mn2+
Pale pink
Oxidation state +2
State colour of Fe2+
Pale green
Oxidation state +2
State colour of Co2+
Pink
Oxidation state +2
State colour of Ni2+
green
Oxidation state +2
State colour of Cu2+
blue
Define a complex
A complex is a central metal atom or ion surrounded by co-ordinately bonded ligands

Define a co-ordinate bond
Covalent bond in which both electrons in the shared part come from the same atom
(In complex, they come from ligand)

Define a ligand
Atom, ion or molecule that donates a pair of electrons to a central transition metal ion to form a co-ordinate bond

Define co-ordination number
no. of co-ordinate bonds that are formed with the central metal ion
Name 2 examples of small ligands
H2O or NH3
If ligands are small (like H2O or NH3), state how many co ordinate bonds can fit around the central metal ion
6
Name an example of a bigger ligand
Cl-
If ligands are large (like Cl-), state how many co ordinate bonds can fit around the central metal ion
4
6 co-ordinate bonds mean an _____ shape
6 co-ordinate bonds mean an octahedral shape
State the bond angles for an octahedral shape
90°
Draw [Fe(H2O)6]2+ (aq)

State the formula of

[Co(NH3)6]3+ (aq)
Draw [Cu(NH3)4(H2O)2]2+ (aq)

4 co-ordinate bonds usually mean a ________ shape
4 co-ordinate bonds usually mean a tetrahedral shape
State the bond angles for a tetrahedral shape
109.5°
Draw [CuCl4]2-

4 co-ordinate bonds can form a ____ _____ shape
4 co-ordinate bonds can form a square planar shape
e.g. cisplatin

State the bond angles for a square planar shape
90°
Some silver complexes have 2 co-ordinate bonds and form a ______ shape
Some silver complexes have 2 co-ordinate bonds and form a linear shape
Draw [Ag(NH3)2]+ (Tollens’ reagent)

State the bond angles for a linear shape
180°
Overall charge on complex ion is its ___ ____ ____
total oxidation state

State how you would work out the oxidation state of a metal ion

Give the oxidation state of the cobalt ion in [CoCl4]2-

Give the oxidation state of the chromium ion in [CrCl2(H2O)4]+

Why must a ligand have at least one lone pair of electrons?
∵ otherwise it won’t have anything to use to form a co-ordinate bond
What are monodentates?
Ligands that only form 1 co-ordinate bond
What are multidentates?
Ligands that form more than 1 co-ordinate bond
e.g. EDTA4- has 6 lone pairs
What are bidentates?
- (multidentate) ligands that can form 2 co-ordinate bonds
- Donates an electron pair from two different atoms

Draw a ethane-1,2-diamine (en) molecule

Draw an ethanedioate (C2O42-) molecule

Name a multidentate ligand that forms 6 co-ordinate bonds with a metal ion

Describe the overall structure of haemoglobin
Haemoglobin contains Fe2+ ions, which are hexa-coordinated (6 co-ordinate bonds) = octahedral structure
Describe the haem part in haemoglobin
- Haem is an iron(II) complex with a multidentate ligand
- 4 co-ordinate bonds come from single multidenate ligand
- 4 nitrogen atoms from same molecule co-ordinate around Fe2+ to form circle
- This part of molecule is called haem
State where the other 2 co-ordinate bonds come from in haemoglobin (i.e. not N)
Other 2 co-ordinate bonds come from protein called globin, and oxygen or water molecule
What does the complex in haemoglobin allow it do?
Complex can transport oxygen to where its needed & then swap it for a water molecule
Explain how haemoglobin can transport oxygen to where its needed & then swap it for a water molecule
- In lungs (O₂ = high), O₂ substitutes water ligand and bonds co-ordinately to Fe(II) ion to form oxyhaemoglobin which is carried around the body in the blood
- When oxyhaemoglobin gets to place where O₂ is needed, oxygen molecule is exchanged for water molecule
Draw Haemoglobin
(with either water or oxygen)

Explain what happens to haemoglobin if CO is inhaled
- Haemoglobin swaps its water ligand for a CO ligand forming carboxyhaemoglobin
- CO = strong ligand & doesn’t readily exchange with oxygen or water ligands ∴ haemoglobin can’t transport oxygen
Complex ions can show _____ isomerism
optical isomerism (type of stereoisomerism)
What is optical isomerism?
Where ion can exist in 2 forms that are non-superimposable mirror images
When do complex ions show optical isomerism?
Happens with octahedral complexes when 3 bidentate ligands (e.g. ethane-1,2-diamine) co-ordinately bond with central metal ion (e.g. nickel)

Cis-Trans Isomers can form in _______ and ______ _____ Complexes
Cis-Trans Isomers can form in Octahedral and Square Planar Complexes
Describe octahedral complexes that show cis-trans isomerism
Octahedral complexes with 4 monodentate ligands of 1 type & 2 monodentate ligands of another type
Octahedral Complexes
When does a trans isomer occur?
If 2 odd ligands are opposite each other

Octahedral Complexes
When does a cis isomer occur?
If 2 odd ligands are next to each other

Describe square planar complexes that show cis-trans isomerism
Square planar complex ions that have 2 pairs of ligands
Square Planar Complexes
When does a trans isomer occur?

Square Planar Complexes
When does a cis isomer occur?

What happens to the 3d orbitals when ligands bond to ions?
Some of the orbitals gain energy which splits the 3d orbitals into 2 different energy levels

Electrons tend to occupy the _____ ____

lower orbitals/ground state
What do electrons need to jump to the higher orbitals (excited states) and where do they get this from?

- They need energy equal to the energy gap, ΔE
- Get this energy from visible light


What affects the size of the energy gap (ΔE)?
- Central metal ion
- Its oxidation state
- Ligands
- Co-ordination number
The larger the energy gap, the _____ the frequency of light that is absorbed
The larger the energy gap, the higher the frequency of light that is absorbed

Explain why the colours of transition metal ions are complement of those that are absorbed
- When visible light hits transition metal ion, some frequencies are absorbed when d electrons jump to higher orbitals/are excited
- Frequencies absorbed depend on size of energy gap (ΔE)
- Rest of frequencies transmitted or reflected
- These frequencies combine to make complementary colour of the absorbed frequencies = colour you see
- e.g. hydrated [Cu(H2O)6]2+ ions
- Absorb “red” light
- Rest of frequencies combine to produce complementary colour = blue
Explain why some compounds appear white/colourless
- If no 3d electron or 3d sub-level is full
- = no electron will jump ∴ no energy absorbed
- ∴ compound = white/colourless
How can the colour of a complex be altered?
By any factors that can affect the size of the energy gap (ΔE)

What is spectroscopy used to find?
The conc. of a solution by measuring how much light it absorbs
Describe how you can use spectroscopy to find concentrations of transition metal ions
- White light shone through filter, that only lets through the colour of light that’s absorbed by the sample
- Light passes through sample to colorimeter
- Calculates how much light was absorbed by the sample
- More conc. coloured solution is = more light it’ll absorb

Describe how you can use light absorption measurement to find conc. of solution of transition metal ions
- Produce a calibration curve
- Involves measuring absorbance of known conc. of solutions & plotting results on a graph
- Then can measure absorbance of your sample & read its conc. off the graph

Ligand Substitution
If ligands are of similar size and the same charge, then the ________ and _____ of the complex ion doesn’t change
If ligands are of similar size and the same charge, then the co-ordination number and shape of the complex ion doesn’t change

Ligand Substitution
If ligands are ______ ____, they’ll be a change in co-ordination number and shape
If ligands are different sizes, they’ll be a change in co ordination number and shape

Ligand substitution reactions can be easily reversed. State when they can’t be.
When new complex ion is more stable than old one
Give 2 examples of when ligand substitution reactions can’t be easily reversed
- If new ligands form stronger bonds with central metal ion than old ligands did
- Multidentate ligands form more stable complexes than monodentate ligands

Explain why enthaply change for a ligand substitution reaction is usually very small
When ligand exchange reaction occurs, strength of co-ordinate bonds broken is often very similar to strength of new co-ordinate bonds being made

Why is this reaction considered irreversible when it is actually reversible?

- Equilibrium lies so far to the right
- [Ni(NH2CH2CH2NH2)3]2+ is much more stable than [Ni(NH3)6]2+
- Not accounted for by an enthalpy change
What explains why multidentate ligands always form much more stable complexes than monodenate ligands?
The chelate effect
Explain what the chelate effect is
- When monodentate ligands are substitued with bidentate/multidentate ligands, the no. of particles in solution ↑
- More particles = greater entropy
- Reactions that result in greater entropy are more likely to occur
Difficult to reverse these reactions ∵ reversing = decrease in entropy

Transition metals can exists in ___ ______ _____
variable oxidation states

Describe how vanadium(V) ions can be reduced
By adding them to zinc metal in an acidic solution
Write the equation for when VO2+(aq) reacts with Zn(s)

Write the equation for when VO2+(aq) reacts with Zn(s)

Write the equation for when V3+(aq) reacts with Zn(s)

What does the redox potential of ion/atom tell you?
How easily the ion/atom is reduced to lower oxidation state
(same as electrode potentials)
Larger redox potential, less _____ ion will be & more likely
it’s to be ______
Larger redox potential, less stable ion will be & more likely it’s to be reduced
Redox potential of an ion _____ always be same as its standard electrode potential
WON’T
What is the redox potential for a transition metal ion, when it changes from a higher to a lower oxidation state, influenced by?
- pH
- ligand
Explain how different ligands affect redox potentials (& make them differ from standard electrode potentials)
- Standard electrode potentials are measured in aqueous solution = aqueous ions will be surrounded by water ligands
- Different ligands may make redox potential larger or smaller depending on how well they bind to a metal ion in particular oxidation state
Explain how different pHs affect redox potentials
- Some ions need H+ to be present in order to be reduced
- Others release OH- ions into solution when they are reduced
- pH of solution affects size of redox potential for these reactions
- Redox potentials will be large in more acidic solutions, making ion more easily reduced
What reaction does Tollens’ reagent use to distinguish between aldehydes and ketones? State the equation

Describe how Tollens’ reagent is prepared
Add ammonia solution to silver nitrate solution to form colourless solution containing complex ion [Ag(NH3)2]+
Describe what happens when aldehyde is added to Tollens’ reagent
- Tollens’ reagent reacts to give silver mirror on inside of test tube
- Aldehyde is oxidised to carboxylic acid, Ag+ ions are reduced to silver metal
Write the equation for when Tollen’s reagent reacts with an aldehyde (RCHO)

Titrations using Transition Element Ions are _____ Titrations
Redox
Titrations with Transition Metals
What can you use the titrations to find out?
How much oxidising agent is needed to exactly react with a quantity of reducing agent
Titrations with Transition Metals
Suggest an oxidising agent you can use
aqueous potassium manganate(VII)
Titrations with Transition Metals
Suggest why aqueous potassium manganate(VII) is used as an oxidising agent
- Contains purple manganate(VII) ions
- Strong acidic conditions are needed for manganate(VII) ions to be reduced
Titrations with Transition Metals
Suggest 2 reducing agents you can use
- aqueous Fe2+ ions
- aqueous C2O42- ion
Titrations with Transition Metals
Describe a method
- Measure quantity of reducing agent using a pipette & add to conical flask
- Using a measuring cylinder, add 20 cm3 of dilute sulfuric acid to flask
- This is in excess
- Add oxidising agent to reducing agent using burette
- Swirling conical flask
- Oxidising agent added reacts with reducing agent
- Reaction continues until all reducing agent is used up
- Next drop added = mixture becomes colour of oxidising agent
- Stop when mixture in flask becomes tainted with colour of oxidising agent (end point) and record volume of oxidising agent added
- Rough titration
- Do some accurate titrations
- Do a few until you get 2 or more reading are within 0.10 cm3 of each other




Why do transition metals and their compounds make good
catalysts?
- ∵ change oxidation states by gaining or losing electrons within d orbitals
- ∴ can transfer electrons to speed up reactions
What is the catalyst used in the Contact Process to make sulfuric acid?
Vanadium(V) oxide
Why is vanadium(V) oxide used as a catalyst in the Contact Process?
- ∵ it’s able to oxidise SO2 to SO3 and it can be reduced to vanadium(IV) oxide
- & then it’s oxidised back to vanadium(V) oxide by oxygen to react all over again
Describe and state the 1st equation that occurs in the Contact Process. Include state symbols.

Describe and state the 2nd equation that occurs in the Contact Process. Include state symbols.

What are heterogenous catalyst?
A catalyst that’s in a different phase from the reactants
i.e. in a different physical state
Where do reactions occur on in heterogenous catalysts?
Occur on on active sites on surface of heterogenous catalyst
Explain why increasing SA of a catalyst increases the rate of a reaction
Increases no. of molecules that can react at same time
What are often used to make the area of catalyst as large as possible?
Support mediums
Explain how support mediums help to minimise the cost of a reaction?
∵ only small coating of catalyst is needed to provide large SA
Name 2 heterogeneous catalysts
- Iron in Haber Process (for making ammonia)
- Vanadium(V) oxide in Contact Process
State the overall equation for the Haber Process. Include the catalyst and state symbols.

State the overall equation for the Contact Process. Include the catalyst and state symbols.

How do heterogenous catalyst work?
By adsorbing reactants onto active sites located on their surfaces
Describe catalyst poisoning
Impurities (in reaction mixture) bind to catalyst’s surface and block reactants from being adsorbed
Explain how catalyst poisoning slows down the rate of a reaction
Catalyst poisoning reduces SA of catalyst available to reactants
Explain why catalyst poisoning increases cost of chemical process
- ∵ less product can made in certain time or with a certain amount of energy
- Catalysts may need replacing or regenerated = costs money
Name a substance that poisons the iron catalyst in the Haber Process
sulfur
Explain how sulfur poisons the iron catalyst in the Haber Process
- Hydrogen in Haber process is produced from methane
- Methane is obtained from natural gas - contains impurities like sulfur compounds
- Any sulfur not removed is adsorbed onto iron forming iron sulfide = stops iron catalysing reaction efficiently
What are homogenous catalysts?
Catalysts that are in the same physical state as reactants
Usually the homogenous catalyst is an _______ catalyst for a reaction between 2 aqueous solutions
aqueous
Describe how homogenous catalysts work
Work by combining with reactants to form an intermediate species which reacts to form products and re-form the catalyst
Why does the enthalpy profile for a homogeneously catalysed reaction contain 2 humps?
2 steps in reaction

Activation energy needed to form intermediates is lower than…
that needed to make products directly from reactants
______ reaction between iodide ions and peroxodisulfate (S2O82-) ions take place ________
Redox reaction between iodide ions and peroxodisulfate (S2O82-) ions take place very slowly
Why does the redox reaction between iodide ions and peroxodisulfate (S2O82-) ions take place very slowly?
- ∵ both ions negatively charged
- Ions repel each other & so its unlikely they’ll collide and react

State the equation for when iodide ions and peroxodisulfate ions react together

Name a catalyst that speeds up the reaction between iodide ions and peroxodisulfate ions
Fe2+ ions
Explain why adding Fe2+ ions speeds up the reaction between iodide ions and peroxodisulfate ions
∵ each stage of reaction involves a positive and a negative ion = no repulsion
Describe and state the 1st equation that occurs when Fe2+ ions is added to iodide ions and peroxodisulfate ions. Include state symbols.
Fe2+ are oxidised to Fe3+ ions by S2O82- ions

Describe and state the 2nd equation that occurs when Fe2+ ions is added to iodide ions and peroxodisulfate ions. Include state symbols.
Newly formed intermediate Fe3+ ions now easily oxidise the I- ions to iodine & catalyst is regenerated

Mn2+ ions _________ the reaction between C2O42– and MnO4–
Mn2+ ions autocatalyse the reaction between C2O42– and MnO4–
Explain what it meant by an autocatalysis reaction
- A product of a reaction that acts as a catalyst for the reaction
- Means that as a reaction progresses and the amount of product increases = reaction speeds up
Write the overall equation for when Mn2+ ions autocatalyse the reaction between C2O42– and MnO4–

Describe and state the 1st equation that occurs when Mn2+ ions is added to C2O42– and MnO4–. Include state symbols.
Mn2+ oxidised to Mn3+ by MnO4- ions

Describe and state the 2nd equation that occurs when Mn2+ ions is added to C2O42– and MnO4–. Include state symbols.
Mn3+ reduced to Mn2+ (re-form catalyst ions) by C2O42- ions

Draw [Cr(en)3]3+ & state its shape and co-ordination number
Octahedral & 6

Draw [Co(en)2Cl2]+ & state its shape and co-ordination number
Octahedral & 6
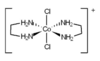
Draw two geometric isomers of [NiCl2(H2O)4]

Draw two geometric isomers of square planar complex [PtCl2(NH3)2]
cis isomer = cisplatin

Draw the two optical isomers of octahedral complex [Fe(C2O4)3]3-

State the charge of :CN- ligand
-1
State the charge of :OH- ligand
-1
Draw the structure of the ethanedioate ion, C2O42-. Explain how this ion is able to act as a ligand. (2)
lone pair(s) on O– / O

Draw the structure of the 1,2-diaminoethane (en)

Give an example of complete ligand substitution when the co-ordination number and shape doesn’t change. Include the colour change & shape.

Give an example of incomplete ligand substitution when the co-ordination number and shape doesn’t change. Include the colour change & shape.

Give an example of complete ligand substitution when the co-ordination number and shape changes. Include the colour change & shape.

Ligand Substitution
Write an equation where a monodentate ligand replaces a monodentate ligand e.g. with [Fe(H2O)6]3+ with CN-

Ligand Substitution
Write an equation where a bidentate ligand replaces a monodentate ligand e.g. with [Cu(H2O)6]2+ and ethane-1,2-diamine

Ligand Substitution
Write an equation where a multidentate ligand (that’s not a bidentate ligand) replaces a monodentate ligand e.g. with [Cr(NH3)6]3+

When using potassium manganate(VII) in redox titrations with iron(II) ions it is essential that the reaction mixture is acidified. Explain why. (1)
ensures all MnO4- reacts to form Mn2+ / stop formation of MnO2 / becomes colourless
Explain why an indicator is not needed in a redox titration with e.g. potassium manganate(VII) & iron(II) ions (1)
It’s self-indicating
Suggest one reason why the colour of potassium manganate(VII) can be a source of error when using a volumetric flask to prepare a standard solution (1)
Difficult to see meniscus
State the equation for when dichromate(VI) ions react with iron(II) ions
Cr2O72- + 14H+ + 6Fe2+ → 2Cr3+ + 7H2O + 6Fe3+
Suggest one reason why electron pair repulsion theory cannot be used to predict the shape of the [CoCl4]2- ion (1)
Too many electrons in d sub-shell
The redox reaction between acidified potassium manganate(VII) and sodium ethanedioate: Sketch a graph to show how the concentration of MnO4− ions varies with time in this reaction. Explain the shape of the graph. (4)
- Starts slowly with low rate
- ∵ -ve ions collide so high Ea
- Rate increases as autocatalyst (Mn2+) forms
- Rate decreases as concentration of MnO4− ions / reactant(s) decreases (OR reactants are being used up)

When the complex ion [Cu(NH3)4(H2O)2]2+ reacts with 1,2-diaminoethane, the enthaply change is appromimately zero. Suggest why. (2)
- Cu-N bonds formed have similar enthaply/energy to Cu-N bonds broken
- And same no. of bonds broken & made
State the colour change for when iron(II) ethanedioate is titrated against potassium managante(VII) (burette)
Colourless to pale pink
∵ tiny excess of MnO4- (manganate(VII)) ions present
Give 2 reasons why the use of a spectrometer is the most appropriate method for measuring the concentration of coloured ions (2)
- Rapid determination of concentrations
- Doesn’t use up any of the reagent
Write an equation for the reaction that occurs between an aqueous solution of aluminium chloride and an excess of aqueous diaminoethane. Describe the appearance of the aluminium-containing reaction product. (3)
2[Al(H2O)6]3+ + 3H2NCH2CH2NH2 → 2Al(H2O)3(OH)3 + 3[H3NCH2CH2NH3]2+
White precipitate


