3.1.1 Atomic Structure Flashcards
_____ take up most of the volume in atoms
Orbtitals take up most of the volume in atoms
Relative mass for an electron
1/1840
What letter represents the mass number?
A
What letter represents the atomic number?
Z
What type of ions have…
No. of electrons < No. of protons
Postive Ions
What type of ions have…
No. of electrons > No. of protons
Negative Ions
What holds the protons and neutrons?
Strong nuclear force
What holds electrons and protons together in atom?
Electrostatic forces of attraction
Why is the strong nuclear force stronger than electrostatic forces?
It overcomes repulsion between protons in nucleus
Strong nuclear force acts only over very ___ distances
SHORT distances (within nucleus)
What decides the chemical properties of an element?
No. & arrangement of electrons decides
Why do isotopes have the same chemical properties? (2)
- ∵ they have same electron configuration
- chemical properties depend on electrons
Isotopes have ___ _____ physical properties
slight different physical properties
Why do isotopes have slight different physical properties?
∵ physical properties depend on mass of atom
19th century: What did John Dalton say atoms were?
- Solid spheres
- Different spheres made different elements
- (All atoms of an element = same mass)
1897: What did J.J. Thomson discover and what did it show?
- Discovered the electron
- Showed atoms weren’t solid and indivisible
- (Model known as ‘plum pudding model’)

1909 - Ernest Rutherford: What did he find out?
Conducted the golden foil experiment:
- Fired positively charged alpha particles at a very thin sheet of gold
- Particles passed straight through gold & only small no. of particles were deflected backwards (pulm pudding model said = alpha particles would be deflected by the positive ‘pudding’ in atom)
- = developed into nuclear model of atom
- Tiny positive nucleus surrounded by ‘cloud’ of negative electrons - most of atom is empty space

What was Niels Bohr’s model & discovery?
- Model: where electrons exist in shells or orbits of fixed energy
- Discovered: When electrons move between shells, electromagnetic radiation (with fixed energy or frequency) is emitted/absorbed

What have modern day scientists discovered & so what did they do?
- Electrons in same shell ≠ same energy
- Bohr model = wrong ∴ they refined it & added sub-shells
- (Isn’t perfect model but it’s simple and explains many experimental observations e.g. bonding & ionisation energy trends)
What are relative masses essentially?
Masses of atoms compared to carbon-12
Define Relative Atomic Mass (Ar) of an element (1x)
Average mass of an atom of an element on a scale where an atom of carbon-12 is 12
Define Relative Isotopic Mass
Mass of an atom of an isotope of an element on a scale where an atom of carbon-12 is 12
Define Relative Molecular Mass (Mr)
Average mass of a molecule on a scale where an atom of carbon-12 is 12
What does a mass spectrometer do and how?
- It determines the mass of separate atoms (or molecules)
- Works by forming ions from sample and then separating them according to the ratio of their charge to their mass
Name the 6 things that happen when a sample is squirted into time of flight (TOF) mass spectrometer
- Vacuum
- Ionisation
- Acceleration
- Ion Drift
- Detection
- Data Analysis
Describe the step vacuum in mass spectrometry (TOF)
Whole apparatus is kept under a vacuum to prevent ions produced from colliding with air molecules
Name the two ways you can ionise your sample in mass spectrometry (TOF)
2 methods:
- Electrospray ionisation
- Electron impact ionisation
Describe the method electrospray ionisation
- A high voltage is applied to a sample in a polar solvent
- Sample molecule, M, gains a proton forming MH+
Describe the method electron impact ionisation
- Sample is bombarded by high energy electrons
- Sample molecule loses an electron = become +1 ions (M+)
Describe the step acceleration in mass spectrometry (TOF)
Positively charged ions are accelerated by an electric field (attracted to negatively charged plate) so = they all have same kinetic energy
Describe the step ion drift in mass spectrometry (TOF)
- Ions enter region with no electric field so they just drift through it
- Lighter ions will drift faster than heavier ions
Describe the step detection in mass spectrometry (TOF) & state how abundance is measured
- Lighter ions travel at higher speeds = reach detector in less time than heavier ions
- Positive ions collected at detector
- Causing current to flow / detected electrically
- Abundance measured: idea that current depends on number of ions hitting detector
Describe the step data analysis in mass spectrometry (TOF)
Signal from detector is sent to a computer which generates a mass spectrum
What does the y-axis of mass spectrum represent?
Abundance of ions
What does the height of each peak give on the mass spectrum?
Relative isotopic abundance
If the sample is an element, what does each line represent on the mass spectrum?
A different isotope of the element
What does the x-axis on the mass spectrum represent?
‘mass/charge’ ratio (m/z)
Describe how to work out the relative atomic mass from mass spectrum (4)
- Spectrum gives relative abundance (of isotopes) & m/z (mass/charge ratio)
- Multiply m/z by relative abundance for each isotope
- Sum these values
- Divide by the sum of the relative iostopic abundances

Why do elements with isotopes produce more than one line in a mass spectrum?
∵ isotopes = different masses
Describe how you can use mass spectrometry to identify elements
You can see if the sample being analysed has the same relative abundances of isotopes

Explain how you use mass spectrometry to identify molecules
mass/charge ratio (of peak) = relative molecular mass of molecule

Electrons have ____ ______ & move around nucleus in certain regions of atom called ____________
Electrons have fixed energies & move around nucleus in certain regions of atom called shells/energy levels
Each shell is given a number called ____ ____ _____
principal quantum number
What is the principal quantum number?
2(n2)
The further away a shell is from nucleus, the _____ its energy & the ____ its principal quantum number
higher its energy + larger its principal quantum number
Electrons in same the shell ___ have same energy
DON’T
Shells divided up into sub-shells which have ____ ______ energies
slightly different
Sub-shells have different no. of orbitals which can hold up to ___ electrons
2


2 electrons in each orbital…
spin in opposite directions
Electrons fill up ___ energy sub-shell 1st
Electrons fill up lowest energy sub-shell 1st

Why do electrons fill orbitals singly before they start sharing?
∵ electrons repel each other
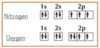
Electron configuration: Give 2 examples of transition metals behaving unusually
Chromium (Cr) & copper (Cu) = donate 1 of their 4s electrons to 3d sub-shell
Write the electron configuration for chromium
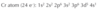
Write the electron configuration for copper
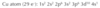
Electron configuration: what happens when transition metals become an ion?
They lose 4s electrons before their 3d electrons

Groups 4-7 can _______ electrons when they form _____ ____
Groups 4-7 can share electrons when they form covalent bonds
Why are the gases in Group 0 inert?
∵ completely filled s & p sub-shells
Why does Chromium (Cr) & copper (Cu) donate 1 of their 4s electrons to 3d sub-shell?
∵ they’re happier with a more stable full or half-full d sub-shell
Define first ionisation energy
Enthalpy change when 1 mole of gaseous 1+ ions is formed from 1 mole of gaseous atoms
Ionisation is a ________ process ∵ you have to put energy in to ionise atom/molecule
endothermic
Write an equation for the first ionisation of oxygen

Name 3 rules about ionisation energies
- Must use gas state symbol (g) ∵ ionisation energies are measured for gaseous atoms
- Always refer to 1 mole of atoms
- Lower ionisation energy = easier it is to remove from ion
Name 3 factors that affect ionisation energy
- Nuclear Charge
- Shielding
- Distance from Nucleus
Describe how nuclear charge affects ionisation energy
More protons in nucleus = more positively charged nucleus is & stronger the attraction for electrons
Describe how shielding affects ionisation energy
As no. of electrons between outer electrons & nucleus increases = outer electrons feel less attraction towards nuclear charge
Lessening of pull of nucleus by inner shells is called shielding (or screening)
Describe how distance from nucleus affects ionisation energy
Attraction decreases rapidly with distance
(i.e. electron close to nucleus = much more strongly attracted than one further away)
What is meant by high ionisation energy?
High ionisation energy = high attraction between electrons & nucleus = more energy needed to remove electron
What provides evidence for shells structure of atoms?
Graph of successive ionisation energies
Within each shell, successive ionisation energies ______
increase
Why does successive ionisation energies increase within each shell?
∵ electrons are being removed from an increasingly positive ion = less repulsion amongst remaining electrons ∴ they’re held more strongly by nucleus

When does big jumps in ionisation energy happen?
When a new shell is broken into = an electron is being removed from shell closer to nucleus

Define second ionisation energy
Enthalpy change when 1 mole of gaseous 2+ ions is formed from 1 mole of gaseous 1+ ions
State the equation for the second ionisation of oxygen

State the equation for the nth ionisation energy

Describe how you can use a successive ionisation energies graph to figure out which group an element belongs to
Count how many electrons are removed before the 1st big jump to find the group number

Describe how you can use a successive ionisation energies graph to predict the electronic structure of elements
Working from right to left, count no. of points there are before each big jump to find how many electrons there are in each shell, starting with the first

Name 2 trends in first ionisation energy
- 1st ionisation energies of elements down a group of periodic table decrease
- 1st ionisation energies of elements across a period generally increase
Explain why ionisation energy decreases down Group 2
- Atomic radius increases/electron removed further from nucleus
- As group is descended more shielding = nucleus’ attraction reduces
Both of these factors = make it easier to remove outer electrons = lower ionisation energy

Explain why ionisation energy increases across a Period (2x)
- Increased nuclear charge (no. of protons is increases = stronger nuclear attraction)
- Extra electrons enter roughly same energy level or similar shielding
Drops between Groups __ and ___ show _____ Structure
Drops between Groups 2 and 3 show Sub-Shell Structure
Describe and explain how aluminium provides evidence for the theory of electron sub-shells
- Aluminium’s outer electron = in 3p orbital rather than 3s
- ∵ 3p orbital = slightly higher energy than 3s orbital
- ∴ electron is found f_urther from nucleus_
- ∵ 3p orbital = slightly higher energy than 3s orbital
- Additonal electron shielding - 3p orbital has additional shielding provided by 3s2 electrons
- Both these factors strong enough to override effect of increased nuclear charge = ionisation energy drops slightly, easier to remove electron

Drops between Groups __ and __ Is due to Electron ______
Drops between Groups 5 and 6 Is due to Electron Repulsion
Describe and explain how phosphorus & sulfur provides more evidence for the eletronic structure model
- (Shielding identical in phosphorus & sulfur atoms + electron is being removed from an identical orbital)
- In phosphorus’s case: electron being removed from singly-occupied orbital
- But in sulfur: electron removed from paired electrons in 3p orbital
- Electron repulsion between 2 electrons = electron easier to remove from pair

Explain why the value of the first ionisation energy of neon is higher than that of sodium (2x)
- Electron removed from a level of lower energy or e– removed from 2p rather than from 3s
- Less shielding
Write the electron configuration for calcium using noble gas symbols

Which of Na+ and Mg2+ is the smaller ion? Explain why (2)
Mg2+
Has more protons with same sheilding
Magnesium exists as three isotopes: 24Mg, 25Mg & 26Mg

24Mg percentage = 80%
26Mg percentage = 10%



