30, Overview of Infection & Immunity 💢 Flashcards
What are all blood cells ultimate derived from?
Pluripotent haematopoietic stem cells
What two lineages can this give rise to?
- Lymphoid
- Myeloid
What cells are derived from the multipotent myeloid precursor?
Megakaryocytes Granulocyte-Monocyte Erythroid
What cells are derived from the multipotent lymphoid precursor?
T cells B cells NK cells
What are some common features of blasts AKA precursor cells ?
- Examples
- Oligodendrocyte precursor cell
- Myeloblast
- Thymocyte
- Megakaryoblast
- Melanoblast
- Lympoblast
- Bone marrow precursor cells
- They have a large nucleus and a small amount of cytoplasm
How does the colour of red cells change as they mature?
- When they are immature they are more blue/purple
- As they mature they become pinker
Which cells produce erthyropoietin (EPO)? What can trigger the production of EPO?
- EPO is mainly produced in the kidneys by the juxtatubular interstitial cells
- It is also produced to a lesser extent by the liver
- EPO production is stimulated by hypoxia
- (Erythropoietin has its primary effect on red blood cell progenitors and precursors (which are found in the bone marrow in humans) by promoting their survival through protecting these cells from apoptosis)
What is the life span of a red blood cell?
120 days
Examples of granulocytes AKA polymorphonuclear proteins (PMNs)
- Basophils
- Eosinophils
- Neutrophils
- Mast cells
What feature allows red blood cells to wriggle through small holes in the capillaries in the spleen?
- As they lack a nucleus, red blood cells have an extensive cytoskeleton meaning that it is very flexible and can fit through small gaps
- As the cells get older, they becomes less flexible and less able to pass through the capillaries into the sinuses in the spleen
- This means that they are more likely to be retained in the spleen and phagocytosed
Define anisocytosis and poikilocytosis
Anisocytosis = red cells show more variation in SIZE than is normal
Poikilocytosis = red cells show more variation in SHAPE than is normal
What can be used as a reference in a blood film to determine whether the red blood cells are microcytic or macrocytic?
Lymphocytes are generally all the same size
Define hypochromia.
The red cells have a larger area of central pallor than normal
NOTE: normal red cells have a central pallor that covers around 1/3 of the red cell diameter
NOTE: hypochromia and microcytosis tend to go together

Define hyperchromia.
The red cells lack a central pallor

State two important types of hyperchromatic cells.
1) Spherocytes
2) Irregularly Contracted Cells

What is responsible for the round shape of the spherocytes in spherocytosis?
It is caused by a loss of cell membrane that is not accompanied by an equivalent loss of cytoplasm
State a cause of spherocytosis.
Hereditary spherocytosis
What usually causes the formation of irregularly contracted cells?
Oxidant damage
Define polychromasia.
An increased blue tinge to the cytoplasm of a cell
What can reticulocytes be stained with?
Methylene blue

State six different types of poikilocytosis.
- Spherocytes
- Elliptocytes
- Fragments
- Irregularly contracted cells
- Target cells
- Sickle cells
What are target cells? State some causes of target cells in the blood film.
- Target cells have an accumulation of haemoglobin in the middle of the central pallor
- It is caused by:
- obstructive jaundice
- hyposplenism
- liver disease
- haemoglobinopathies

State two causes of eliptocytosis.
- Hereditary eliptocytosis
- Iron deficiency (anaemia)
What biochemical phenomenon causes the sickling of red blood cells in Sickle Cell anaemia?

Polymerisation of haemoglobin S when present in a high concentration
What is another name for fragments?

Schistocytes
State two different ways in which red blood cells can clump together and describe why they happen.
-
Rouleaux (like a stack of coins)
- caused by a change in plasma proteins pushing the red cells together
-
Agglutinates – irregular clumps
- caused by antibodies on the cell surface making the cells stick together
What is a Howell-Jolly Body and what is it usually caused by?
- This is a nuclear remnant in the red cells
- This is most commonly caused by a lack of splenic function (the spleen should remove these tiny bits of nuclear material)

Which cytokines are important in the differentiation of myeloblasts to granulocytes and monocytes?
- Granulocyte colony-stimulating factor (G-CSF)
- Macrophage colony-stimulating factor (M-CSF)
- Granulocyte-macrophage colony stimulating factor (GM-CSF)
- Interleukins

How long do neutrophils survive for in the circulation?
7-10 hours
What is the main role of eosinophils?
Parasitic infections

Describe the shape of the nucleus of an eosinophil.
Eosinophils have a bilobed nucleus
What is the main role of basophils?
They are involve in the allergic response

Describe the appearance of basophils.
- They have lots of dark blue dots in the cytoplasm
- Often there are so many blue dots that you can’t even see the nucleus
- Granules contain heparin (anticoagulant) and histamine (vasodilator)

Describe the appearance of monocytes.
- They have a kidney bean shaped nucleus
- They are large

Other than phagocytosis, what other roles do macrophages play?
- Adapative immunity
-
Antigen presentation
-
After ingestion, display of antigens to a MHC class II molecule and secretion of IL-12
- M1“killer” macrophages acitvated by LPS (on bacterial cell wall)
- Secrete high levels of IL-12 and low levels of IL-10
- Stimulates type 1 helper T cells (TH1 cells) to proliferate
-
After ingestion, display of antigens to a MHC class II molecule and secretion of IL-12
-
O2-dependent killing of facultative intracellular pathogens
- e.g. Mycobacterium tuberculosis, Leishmania, requires activation by IFN-γ
-
Clearing of virus from blood
- Production of type-I interferons (α/ß)
-
Antigen presentation
-
Tissue homeostasis:
- Bone remodelling
- Muscle regeneration
- Wound healing
- Pathogenic role in chronic inflammation
- Iron haemostasis
- Erthrocytes have a lifespan of 120 days so are constnatly being destroyed by macrophages in the spleen and liver
- Iron released from haemoglobin is either stored internally in ferritin or released into the circulation via ferroportin
How long do platelets survive for in the circulation?
10 days
What term is used to describe having too many white blood cells?
Leucocytosis

What term is used to describe having too many platelets?
Thrombocytosis

Describe the appearance of an atypical lymphocyte.
- An atypical lymphocyte will have a large nucleus and a large amount of faint cytoplasm
- Large irregular atypical lymphocytes seen in the peripheral blood of a patient with infectious mononucleosis.
- The indentation of the cytoplasm of the lymphocyte (arrows) by red blood cells gives rise to the classic “Dutch skirt” appearance of the border

Define granulopoesis and its stages.
- Definition of granulopoesis
- Production of granulocytes
- Granulocyte is a type of white blood cell that has multilobed nuclei usually containing three lobes and a signficiant amount of cytoplasmic granules within the cell
- Examples
- Neutrophils
- Oesinophils
- Basophils
- Mast cells
- Production of granulocytes
- Stages
- Pluripotent haemopoietic stem cell
- Myeloblast
- Promyelocyte
- Eosino/neutro/basophilic myelocyte
- Metamyelocyte
- Band cell (stab cell)
- Granulocytes (eosino/neutro/basophil)

What can cause the atypical appearance of a lymphocyte in a blood film?
-
Viral infection
-
Glandular fever AKA infectious mononucleosis
- Caused by Epstein-Barr virus
-
Symptoms
- Fever
- Sore throat
- Enlarged lymph nodes in the neck
- Tiredness
-
Complications:
- Swelling of the liver or spleen
-
Treatment
- Drinking enough fluids
- Getting sufficient rest
- Pain medication
-
Glandular fever AKA infectious mononucleosis
What is ‘left shift’?
- Left shift or blood shift is an increase in the number of immature leukocytes in the peripheral blood, particularly neutrophil band cells
- Description of blood smear:
- Dark blue nucleus is less segmented than the mature neutrophil on the right
- Caused by:
- active infection
- hypoxia
- shock

What is toxic granulation? What can cause it?
- Dark coarse granules found in granulocytes
- Granules composed of peroxidase and acid hydrolase
- Suggestive of an inflammatory process
- Causes
- Bacterial infection
- Sepsis
- Tissue necrosis
- Feature of normal pregnancy
What is hypersegmentation of neutrophils? What can cause it?
- An increase in the average number of neutrophil lobes
- presence or 5 or more lobes
- Causes
- Vitamin B12 deficiency
- Folic acid deficiency

What process during maturation of the megakaryocytes is important for the formation of platelets?
Granulation

How many platelets are produced by one megakaryocyte?
4000
Broadly speaking, how do Cytotoxic T Lymphocytes kill infected cells?
Inducing apoptosis
What are the two mechanisms by which a cytototoxic T cell (Tc) kill cells?
Granzyme + perforin
- perforin makes a pore in the cell membrane through
- granzyme can enter and trigger apoptosis
-
Cell surface interaction between the Tc and the infected cell
- Fas ligand (CD95L) on T cell binds to Fas receptor (CD95) on infected cell
- When Fas has been engaged – it releases CASPASES
- Both pathways upregulate CASPASES which drives apoptosis
What are the four main effector functions of CD4+ T Lymphocytes?
-
Macrophage activation
- Production of hydrolytic enzymes
- OR formation of a multinucleate giant cell
- Tuberculosis
- B cell activation
-
Delayed type hypersensitivity
- Secretion of IL-12 stimulates the proliferation of further CD4+ and CD8+ cells
- Regulation
What are the two phases involved in Delayed Type Hypersensitivity?
-
Sensitisation
- Initial exposure to the antigen
- T-cell mediated
- Develops slowly as cells accumulate
- Initial exposure to the antigen
-
Effector
- Delayed response
What are the five T helper cell subsets? What are their functions?
-
Th1
- inflammatory responses
-
Th2
- boosts anti-multicellular organism responses
-
Th17
- important in control of bacteria
-
Follicular T helper cells
- essential for generation of isotype-switched antibodies
-
Treg
- regulation of T cell responses

What is the main difference between T cell memory and B cell memory?
T cell memory doesn’t undergo isotype switching or affinity maturation – it does not get better
What is the basic sequence of events that occur during a viral infection?
1) Innate Immune System
* There is a rise in type 1 interferon and a rise in NK cells that flattens out the viral replication
2) Adaptive Immune System
- There is a rise in Cytotoxic T lymphocytes and antibodies, which allows the complete removal of virus from the body.
- Rise in lymphocyte count
State some differences between innate and adaptive immune responses.
- The innate immune response is present from birth.
- It is not very specific and it is fast acting.
- Innate immunity relies on pre-formed and rapidly synthesised components. (Adaptive immune response is the opposite.)
State the two types of triggers of the innate immune response and give an example of each.
-
Damage associated molecular patterns (DAMPs)
- high extracellular ATP
-
Protein associated molecular patterns (PAMPs)
- bacterial cell wall components
What does the acute phase response respond to?
It is an inflammatory response to tissue damage.
What is a main clinical feature of the acute phase response and what causes it?
Fever – caused by Interleukin-1
What are the acute phase proteins and what do they do?
-
C-reactive protein & serum amyloid protein
- they both bind to bacterial cell wall components
- opsonisation
-
Mannan-binding lectin
- binds to mannose, which isn’t commonly found in mammalian cells
- activates mannan-binding lectin pathway of complement activation
- These are soluble PRRs which, once bound, helps activate the complement system
What are the five classes of immunoglobulin and what are their distinct features?
- M
- pentamer
- has 10 binding sites so is good at agglutinating pathogens.
- involved in the primary immune response
- G
- monomer
- 75% of serum Ig
- passes from mother to foetus
- involved in the secondary immune response.
- E
- monomer
- binds to basophils and mast cells and aids degranulation
- involved in immune response
- A
- dimer
- found on mucosal surfaces
- has a secretory component to resist degradation by proteases found in the mucosa
- D
- monomer
- very low serum concentration
- involved in B lymphocyte signalling
How do antibodies kill viruses?
-
Opsonisation
- Make them more easily phagocytosed
-
Neutralisation
- Binding and preventing entry
- Activating complement
-
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
- Coating in antibody means that infected cells are more easily killed by NK cells
What is the difference between B cell receptors and T cell receptors?
- B cell receptors are membrane-anchored forms of the antibody that the B cell will secrete if activated.
- It can bind to intact antigens.
- T cell receptors can only bind to processed antigens, which are presented on MHC molecules.
Describe the process of clonal selection.
Lymphocytes circulate through the lymph until they meet its complementary antigen. When the lymphocyte meets its antigen it will bind and become activated and begin to replicate itself. This is called clonal expansion.
What are the three main types of antigen presenting cell and what do they do?
- 3 main types:
- Macrophages
- Dendritic Cells
- B cells
- They pick up antigens and move to the lymph nodes where circulating lymph nodes can find the antigens.
What are the primary lymphoid organs?
Bone marrow and thymus
What are the secondary lymphoid organs?
- Lymph nodes
- Spleen
- Mucosa-Associated Lymphoid Tissue (MALT)
What is the difference between the types of epitopes recognised by B cells and T cells?
- T cells = sequences
- B cells = structure (tertiary)

Describe the structure of the B cell receptor and how it transmits signals into the cell.
- The BCR is a membrane-anchored antibody
- It is associated with two transmembrane domains called Ig-alpha and Ig-beta which have cytoplasmic tails that are long enough to transmit a signal to the inside of the cell
- Antigen binding to the BCR causes a conformational change, which drives signaling via the Ig-alpha Ig-beta heterodimer
What is the process by which B cells and T cells generate the variety in their receptors/antibodies?
Immunoglobulin Gene Rearrangement
Describe the generation of variation in the light chain.
- There are 70 different V and J regions
- The B cell begins with germline DNA and it cuts out various V and J regions at random leaving only a few
- This means that there is a large number of different combinations of segments forming a large number of different antigen specificities
- Different splicing patterns give rise to more variation
Describe the generation of variation in the heavy chain.
Gene rearrangement is the same – the only difference is that the heavy chain also has a D region and has several different constant regions (determines class)
What enzyme is involved in the removal of unused segments of DNA?
V(D)J Recombinase
What gene encodes for VDJ recombinase and what disease is caused by the deficiency of this gene/enzyme?
- Recombination activating gene (Rag gene)
-
Severe combined immunodeficiency (SCID)
- Primary immunodeficiency
- Greatly increased risks of life-threatening infection
- Persistent coughs and colds, thrush (caused by yeast candida)

What determines the class of the immunoglobulin?
The constant region of the heavy chain

In what order does the gene rearrangement take place?
The heavy chain undergoes rearrangement before the light chain

What three things can happen to B cells once they’ve recognised their antigens?
- Become plasma cells
- Become memory cells
-
Affinity maturation
- Somatic hypermutation
- Mutations in the complementarity-determining regions (CDR)) (variable, antigen-binding coding sequences)
- Clonal selection
- Somatic hypermutation
What is the general rule about B cell and T cell activation?
- It needs co-stimulation to be activated – antigen alone is not enough
- T-cell example
- First signal
- Antigen specific
- Interaction of TCR with peptide-MHC complex on membrane of antigen presenting cell
- Second signal (co-stimulatory signal)
- Antigen non-specific
- CD28 on T-cells interacts with CD80 and CD86 on membrane of APC
- First signal
- B-cell example (continued)
- Latter case induces recognition of antigen-specific Th cells (CD4+) leading to activation of the B cell through binding of TCR to MHC-antigen complex
- Synthesis and presentation of CD40L on Th cells which binds to CD40 on the B-cell and thus the Th co-stimulates the B-cell
What are the two pathways by which B cell production is achieved?
T dependent and T independent
Describe the T independent pathway.
- This is associated with long polysaccharides with a repeating subunit
- The repeating unit can bind to several BCRs and drive cross-linking
- There will also be PAMPs such as LPS that provide co-stimulation
Describe the T dependent pathway.
- Antigen presenting cells (APCs) take up the antigen at the same time B cells process and present the antigen on MHC Class II
- APCs = B-cells, dendritic cells, macrophages,
- Antigen presentation is essential for specificity of response to intracellular and extracellular pathogens
- Dendritic cells also present the SAME antigen on MHC Class II to a T helper cell
- The T helper cell becomes activated and undergoes clonal selection
- The T helper cell then moves to the lymph nodes, comes into contact with the B cell and activates it

Describe the process of immunoglobulin isotype switching AKA class switching
- T helper cells (once bound to the B cell) can release various cytokines – depending on the cytokine released, the immunoglobulin class can be switched
- Dependent on activation induced cytidine deaminase (AID)
- Recombination within the cluster of C genes that cuts out the previously expressed C genee
- Importance seen in hyper-IgM syndrome

What drives the improvement of the immune response between primary and secondary exposures?
Somatic Hypermutation and Affinity Maturation
Describe the process of somatic hypermutation.
- Point mutations are induced in the VDJ regions by activation-induced cytidine deaminase (AID) which cause slight conformational changes in the antigen-binding site
- AID also involved in class switch recombination
- If the change is beneficial and improves the binding between antibody and antigen then it survives
- Otherwise the B cells are killed by apoptosis
What are the three main ways in which the innate immune system can detect pathogens?
PAMPs, DAMPs and missing self

What are the main professional phagocytic cells? List some important features.
-
Granulocytes AKA polymorphoncuelar leukocytes
- Features
- Usually three lobes
- Produced via granulopoesis in the bone marrow
- Examples (NEBM)
-
Neutrophils
- 70% of circulating WBCs
- Eosinophils
- Basophils
-
Mast cells
- Toll-like recpetors
-
Neutrophils
- Features
-
Monocytes/Macrophages
- signal infection by releasing cytokines
-
Dendritic cells
- Present in the tissue in contact with the extenral environment
- Skin
- Inner lining of the nose
- Lungs
- Stomach
- Intestines
- Present in the tissue in contact with the extenral environment

Describe the process of diapedesis - how neutrophils move out of the blood vessels and into tissue towards a pathogen
- Chemoattraction
- Due to infection, activated macrophages release chemokines such as IL-1, TNFα and chemokines
- This causes the endothliel cells of blood vessels near the site of infection to express cellular adhesion molecules including selectins
- Rolling adhesion
- Like velcro, carbohydrate lignads on the circulating leukocytes bind to selectin molecules on the inner wall of the vessel with marginal affinity
- This causes the leukocytes to slow down and begin rolling along the inner surface of the vessel wall
- During this rolling motion, transitory bonds are formed and broken between selectins and their lignads
- P-selectin and P-selectin glycoprotein ligand-1
- Tight adhesion
- At the same time, chemokines released by macrophages activate the rolling leukocytes and cause surface integrin molecules to switch from the default low-affinity state to a high-affinity state
- In the activated state, integrins bind tightly with complementary receptors on endothelial cells with high affinty
- This causes the immobilization of the leukocytes
- (Endothelial) Transmigration
- The cytoskeletons of the leukocytes are reo-organised in such a way that the leukocytes are spread out over the endothelial cells
- Leukocytes extend pseudopodia and pass through the gaps between endothelial cells
- Platelet endothelial cell adhesion molecules (PECAM) proteins found on the leukocyte and endothlial cell surfaces, interact and effectively pull the cell through the endothelium
- Once through the endothelium, the leukocyte must penetrate the basement membrane
- Proteolytic digestion
What are the two main opsonins for neutrophils?
- Antibodies
- Complement proteins
- C1q
- Combines along with C1r and C1s to form C1 complex
- C3b
- C1q

Describe the action of antibodies and complement proteins as opsonins
- Antibodies bind to antigens on the cell surface of pathogens.
- Complement glycoproteins bind directly to the surface of the pathogen.
- These act as adaptors and can then bind to the neutrophil, activating it and stimulating phagocytosis.
What are the mechanisms by which neutrophils kill phagocytosed pathogens?
- Oxygen-dependent
- Respiratory burst
- Rapid release of reactive oxygen speciies
- NADPH oxidase produces superoxide which spontaneously recombines with ohter molecules to produce reactive free radicals
- O2 –> O2- –> H2O2
- Rapid release of reactive oxygen speciies
- Reactive nitrogen intermediates
- Respiratory burst
- Oxygen-independent mechanisms
- LL-37
- Cathelcidin antimicrobial peptides
- Defensins
- Tumour necrosis factor
- Lysozyme
- Hydrolytic enzymes
- LL-37

What are cytokines? List some characteristics.
Cytokine are small secreted proteins that act as messengers. They are short-lived.
Give some examples of cytokines.
Interferons, Interleukins, Growth factors, chemokines, TNF
What are the three ways in which cytokines can act?
- Paracrine
- Endocrine
- Autocrine
Describe the onset and consequences of septic shock.
- Infection causes a massive release of alarm cytokines by activated macrophages (TNF-alpha and IL-1)
- Low blood pressure + Increase in vascular permeability
What is the ‘Complement’ system?
- It’s a system of soluble glycoproteins that enhances the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promote inflammaiton and attack the pathogen’s cell membrane
- It is part of the innate immune system which is not adaptable and does not change during an indiviudal’s lifetime
What are the three ways in which the complement system is activated? Explain how exactly they activate compliment.
-
Classical pathway
- Lysophosphatidylcholine on dead or dying cells is recognised by C-reactive protein
- Activates complement system via C1q
-
Alternative pathway
- C3b binds directly to a microbe
- Results in a cascade and in the production of fluid-phase C3 convertase (serine protease) resulting in proteolytic cleaving
- Lectin pathway
- Mannose-binding lectin recognises mannose residues (found only on pathogens)
- Activates C4 and C2

What happens to the cleaved fragments during the complement cascade?
They are pro-inflammatory molecules, which can bind to receptors on mast cells and cause degranulation giving rise to an inflammatory response.

Other than lysis and opsonisation, what are the two other roles of complement?
-
Activation of the inflammatory response
- by binding to mast cells and macrophages
-
Clearance of immune complexes
- antibody-antigen complexes must be removed before they cause inflammation of blood vessels

What are the two types of mast cell?
- Mucosal
- Connective tissue

What can activate mast cells?
-
Anaphylatoxins
- Examples
- C3a
- C4a
- C5a
- Result
- Degranulation of endothelial cells, mast celsl and pahgocytses
- Smooth muscle cell contraction e.g. bronchospasm
- Increase in the permeability of blood capillaries
- C5a indirectly mediates chemotaxis
- Examples
Describe, in full, a typical inflammatory response to a bacterial pathogen.
- Bacterial infection will firstly activate tissue resident macrophages, which begin producing alarm cytokines and chemokines.
- Macrophages resident in the infected tissues are generally the first cell to sense an invading microorganism.
- If neutrophils are the short-lived infantry of innate immunity, then macrophages are the long-lived commanders who provide warning to other cells and orchestrate the local response to infection
- These cytokines recruit neutrophils and lymphocytes to the area of infection.
- Complement is activated by the classical or alternative pathways
- Classical because lysophosphatidyl choline from dead or dying cells activate C-reactive protein which activates C1q
- Alternative because C3b binds directly to microbe resulting in a cascade and the activation of fluid-phase C3 convertase
- All of which lead to membrane attack complex
- The pro-inflammatory products of complement bind to mast cells and cause degranulation leading to an inflammatory response.
- C3a
- C4a
- C5a
What acute phase proteins are involved in the systemic acute phase response?
- C-reactive protein
- Detecting lysophosphatidylcholine on dead or dying cells
- Mannan-binding lectin
- Detecting mannose exclusively on pathogens
- Fibrinogen
- Complement e.g. C3a, C4a and C5a
State some basic features of NK cells.
- They are large cytotoxic lymphocytes
- They are granular
- Secrete interferon gamma

Describe how NK cells communicate with target cells.
- They don’t have antigen specific receptors.
- Instead they have activating and inhibitory receptors and depending on the balance between the two signals they decide whether to attack the cell.

What are the two types of target cell recognition by NK cells? Explain how they work.
-
Missing self
- When infected, cells will downregulate the expression of MHC Class I, which acts as an inhibitory signal
- The loss of the inhibitory signal means that NK cells are more likely to kill the target cells.
-
Induced self
- Cells will change the pattern of their self-proteins due to stress.
- These stress-induced patterns will be recognised by activating receptors on NK cells and lysed.

What is the lymphoid lineage?
- Lymphoid cells are lymphocytes
- T cells
- B cells
- NK cells
Which cells come under the myeloid lineage?
- Erythrocytes
- Megakaryocytes
- Large bone marrow cell with a lobated nucleus
- Responsible for the production of blood thrombocytes (platelets)
- Monocytes & macrophages
- Eosinophils
- Neutrophils
- Basophils
- Dendritic cells
Describe how the appearance of white cells changes as they develop.
They become smaller and their cytoplasm becomes clearer.
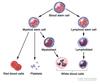
- Which factors stimulate the following cell lines:
- Lymphoid
- Myeloid
-
Lymphoid
- IL-2
- IL-7
-
Myeloid
- IL-3
- Granulocyte colony stimulating factor (G-CSF)
- Monocyte colony stimulating factors (M-CSF)
- Erythropoietin (for erythrocyte)

Define Leukaemia.
- A malignant progressive disease in which the bone marrow and other blood-forming organs produce increased numbers of immature or abnormal leukocytes.
- This leads to suppression of the production of other blood cells such as erythrocytes, granulocytes and platelets.

Define lymphoma
- A group of blood cell tumours that develop from lymphatic cells
- Location
- Tonsils
- Spleen
- Thymus
- Bone marrow
- Lymph node
- Location
- If the disease is mainly in the lymphatic tissue then it is lymphoma.
- If it is mainly in the blood it is leukaemia.

Define multiple myeloma
- A malignant disease of the bone marrow characterised by two or more of the following criteria:
- The presence of an excess of abnormal plasma cells in the bone marrow
- Produce abnormal antibodies causing kidney damage and overly thick blood
- Typical lytic deposits in the bones on X-ray, giving the appearance of holes
- The presence in the serum of abnormal gammaglobulin, usually IgG
- The presence of an excess of abnormal plasma cells in the bone marrow

Broadly speaking, what can cause an increase in white blood cell count?
- Increased white blood cell production
- Increased white blood cell survival
What two broad categories of diseases can cause an increase in white blood cell count?
- Reactive
- Response to infection or inflammation
- Primary
- Malignancy
What is the difference in the type of white blood cell seen in the peripheral blood of someone with an infection/inflammation (reactive) and someone with a malignancy (primary)?
- Reactive (infection)
- only mature white blood cells
- Primary (cancer)
- mature AND immature white blood cells present
Where does the mutation occur in chronic myeloid leukaemia?
GM-CFC phase (granulocyte-monocyte colony forming cell)
If there are only immature cells in the blood film with low haemoglobin and low platelets, what would you suspect?
Acute leukaemia

- What are the normal ranges of:
- Haemoglobin
- Haematocrit
- Erthryocytes
- Leucocytes
- Neutrophils
- Lymphocytes
- Monocytes
- Eosinophils
- Basophils
- Platelets
-
Haemoglobin
- 120 - 160 g/L
- Haematocrit
- 45%
-
Erthryocytes
- 5 x 1012 / L
-
Leucocytes - (7x 109 /L)
-
Neutrophils (NLMEB)
- 40-70%
- 2.8-4.9x 109/L
-
Lymphocytes
- 20-40%
- 1.5-2.8x 109/L
-
Monocytes
- 6%
- 0.42 x 109/L
-
Eosinophils
- 3%
- 0.21 x 109/L
-
Basophils
- <1%
- <0.07x 109/L
-
Neutrophils (NLMEB)
-
Platelets
- 150-400 x 109/L
- 1.5 M - 4.5 M/L
What can cause an elevated lymphocyte count?
- Viral infections
- Chronic lymphocytic leukaemia
What is the lifespan of a neutrophil?
- Hours in the peripheral blood
- 2-3 days in the tissues
What proportion of neutrophils are marginated?
Around 50% of neutrophils in the circulation have marginated meaning that they have stuck to the wall of a damaged vessel (this means that they are NOT counted in the full blood count)

Describe the differences in the appearance of neutrophils in infection compared to leukaemia.
- Neutrophils in infection are granular (show toxic granulation)
- Neutrophils in leukaemia do not have granules and do not look toxic.

What else would be present in the blood film of someone with leukaemia that would not be present in someone with an infection?
- Myelocytes and metamyelocytes – these precursors would not be found in the peripheral blood of someone responding to infection

State some causes of neutrophilia.
- Infection
- Inflammation
- Physical stress
- Adrenaline
- Corticosteroids
- Cortisol
- Underlying neoplasia
- Malignant neutrophilia
- Myeloproliferative disorders
- Chronic myeloid leukaemia
What types of infection cause neutrophilia?
- Bacterial
- Fungal
- Certain viral infections
- Generally: if the neutrophil count is low but there are other features of infection, then you can deduce that it’s a viral infection

State some infections that characteristically do NOT produce neutrophilia.
- Brucella
- Gram negative coccus
- Zoonotic transmission
- Typhoid
- Salmonella enterica
- Gram negative rod
- Many viral infectionsa
State some reactive causes of eosinophilia.
- Parasitic infection
- Allergic diseases
- Asthma
- Neoplasms
- Hodgkin’s and Non-Hodgkin’s
- Hypereosinophilic syndrome
State a malignant cause of eosinophilia.
- Hodgkin’s leukaemia
- Non-hodgkin’s leukameia
- Malignant chronic eosinophilic leukaemia
- Incredibly rare
What would you see in the chest X-ray of someone with Hodgkin’s lymphoma?
Increased mediastinal mass

What can cause monocytosis?
RARE but it is seen in certain chronic infections and primary haematological disorders
- Bacterial
-
Tuberculosis
- Mycobacterium tuberculosis
- Aerobic
- Can appear gram positive or negative due to stains being unable to penetrate the waxy coating of mycolic acid
-
Brucella
- Gram negative coccus
- Zoonotic transmission
- Typhoid
-
Salmonella enterica
- Gram negative rod
- Sarcoidosis
- Abnormal collections of inflammatory lumps known as granulomas
-
Tuberculosis
- Viral
-
Varicella zoster
- One of the 8 herpes viruses known to infect humans
- Causes varicella (chicken pox)
-
Varicella zoster
- Neoplasia & cancer
- Chronic myelomonocytic leukaemia
- Myelodysplastic syndrome (MDS)
Describe the appearance of chronic lymphocytic leukaemia on a blood film.
- The lymphocytes have a typical appearance – big nucleus + little cytoplasm
- They are mature lymphocytes
- This appearance can also be present in autoimmune and inflammatory conditions

Describe the appearance of acute lymphoblastic leukaemia on a blood film.
- There are immature lymphoblasts
- They are much larger than the mature lymphocytes
- Within the large nucleus you can see the nucleolus (showing that the cell is immature)

Describe the difference in the expansion of lymphocytes in secondary (reactive) lymphocytosis compared to primary lymphocytosis.
- Secondary lymphocytosis = polyclonal expansion
- Natural mode of adaptive immune response that ensures a single antigen is recognized and attacked through its epitopes by multiple clones of B cell
- Primary lymphocytosis
- Cancer
State some causes of reactive lymphocytosis.
- Reactive lymphocytosis = cytoxic (CD8+) T-cells cells that have become large as a result of antigen stimulation
- Infection
-
Epstein-Barr virus
- Best known as the cause of infectious mononucleosis AKA glandular fever
- Enlarged tonsils
- Toxoplasma gondi
- Transmitted by dog/cat fecal matter
- Infectious hepatitis
- Rubella
- Herpes infections
-
Epstein-Barr virus
- Autoimmune disorders
- Neoplasia
- Sarcoidosis
- abnormal collections of inflammatory cells that form lumps known as granulomas

What do you see in the blood film of someone with mononucleosis syndrome?
- Atypical lymphocytes
- They look similar to immature lymphocytes but they aren’t very round an its cytoplasm extends between surrounding cells
- The nucleus of the cell lacks nucleoli
- This is typical of glandular fever
- So if there is a high WCC and you find these reactive-looking lymphocytes you can suspect that it is a reactive, infection-induced lymphocytosis

What is glandular fever caused by?
- Epstein-Barr virus infection of the B-lymphocytes via the CD21 receptor
- Infected B-cells proliferate and express EBV associated antigen
- There is a cytotoxic T-lymphocyte response
- Acute infection is resolved leading to life-long sub-clinical infection

What is the usual cause of lymphocytosis in elderly people?
Chronic lymphocytic leukaemia (CLL)

Explain how light chain restriction can be used to distinguish between causes of lymphocytosis.
- In reactive lymphocytosis, there will be polyclonal expansion of the lymphocytes meaning that the light chains of the antigens produced by B cells and the B cell receptors will have a 50:50 kappa and lambda divide
- In primary lymphocytosis, there will be a monoclonal expansion so you will get kappa or lambda restriction meaning that all the light chains are of one type

Normally, B and T cells will undergo gene rearrangement in the TCR and Ig genes in a process called affinity maturation. How is this different in primary monoclonal proliferation of B and T cells?
With primary monoclonal proliferation, all the daughter cells carry identical copies of Ig genes or TCR genes


