3 Organics 1 Flashcards
What is crude oil?
A mixture of hydrocarbons
(which have different boiling points!)
What is a hydrocarbon?
A molecule containing carbon and hydrogen only
Why is crude oil considered to be a non-renewable energy source?
Crude oil takes many million of years to form in the Earth’s crust through heat and high pressures.
It does not renew itself at a sufficient rate for sustainable use
Name the first four hydrocarbon in the alkane homologous series.
(Monkeys eat peeled bananas!)
methane
ethane
propane
butane
What is an alkane?
A hydrocarbon with single C-C bonds only- no C=C double bonds
What is the general formulae for alkanes?
CnH2n+2
What is the general formulae for alkenes
CnH2n
What is the name for C8H18 and what homologous series is it from?
octane
it is an alkane
Alkanes only have a single bond between the carbon atoms.
What effect does this have on their reactivity?
What name is used for this sort of hydrocarbon?
Large number of hydrogen atoms blocking the C-C bond makes it difficult to get to and makes them less reactive.
They are said to be saturated with hydrogen
What is the name for C7H14 and what homologous series is it from?
heptene
It is an alkene
Name the two alkanes with 9 and 10 carbons and give their molecular formulae
Nonane - C9H20
Decane - C10H22
How do molecules in a particular homologous series relate?
- they have trends in physical properties
- they have similar chemical properties
- they have the same functional group
- they differ by CH2 groups
What is the display formula for methane?

Alkenes have a double bond between the carbon atoms.
What effect does this have on their reactivity?
What name is used for this sort of hydrocarbon?
There are fewer hydrogen atoms blocking the C=C bond. This makes it easy to get to and makes them more reactive.
They are said to be unsaturated
(They are not saturated with hydrogen!)
Alkanes only have a single bond between the carbon atoms.
What effect does this have on their reactivity?
What name is used for this sort of hydrocarbon?
Large number of hydrogen atoms blocking the C-C bond makes it difficult to get to and makes them less reactive.
They are said to be saturated with hydrogen
Name the two alkanes with 5 and 6 carbons and give their molecular formulae
Pentane C5H12
Hexane C6H14
Name the two alkanes with 9 and 10 carbons and give their molecular formulae
Nonane - C9H20
Decane - C10H22
Name the two alkanes with 7 and 8 carbons and give their molecular formulae
Heptane - C<span>7</span>H<span>16</span>
Octane - C<span>8</span>H<span>18</span>
Name the two alkanes with 9 and 10 carbons and give their molecular formulae
Nonane - C9H20
Decane - C10H22
What is the display formula for pentane?

What happens to the intermolecular forces as you go down the alkane group?
How do you know?
There are more intermolecular forces down the alkane group
The boiling point increases down the group- more energy needed to overcome the intermolecular forces.
What is the structural formulae for ethane?
CH3CH3
What is the trend in volatility in the alkane group?
Hydrocarbons are more volatile the shorter their chain.
What is the structural formulae for propane?
CH3CH2CH3
What property allows hydrocarbon fractions to be separated?
Name the method used to separate them.
The hydrocarbon fractions have different boiling points
They are separated using fractional distillation
What happens to the viscosity as the carbon chain increases in length?
Viscosity increases as chain length increases
Name the main fractions from crude oil
Hint: there are seven!
Refinery gases
Gasoline
Naptha
Kerosene
Diesel
Fuel Oil
Bitumen
What happens to the bointing point as the carbon chain increases in length?
Boiling point increases as the carbon chain increases
methane is a gas, petrol is a liquid at room temperature
What is an isomer?
Compounds with the same molecular formula but different structures
What happens to the colour as the carbon chain increases in length?
The hydrocarbon gets darker in colour as you increase the carbon chain

What is the addition reaction for propene and bromine water?

Why do you think alkenes are more ‘useful’ in the chemical industry?
They have C=C and so are more reactive
They can be used to make polymers and other chemical products
What would you observe if methane is reacted with bromine in the presence of UV light?
Orange to colourless after about 20 minutes
What type of reaction is this?

Substitution
The hydrogen was substituted with the bromine atom
What type of reaction is this?

addition reaction
double bond is broken and two bromine atoms are added to either side of this
What is the test for alkenes?
add bromine water
turns orange to colourless
alkanes need UV light for 20 minutes to turn colourless

What is combustion?
The chemical reaction of a fuel and oxygen
Complete combustion will produce carbon dioxide and water
the reaction is exothermic
What are the main products for the incomplete combustion of methane?
carbon monoxide, carbon dioxide and water

What is the reaction for complete combustion of methane?

Why is carbon monoxide dangerous?
It is toxic
- it binds irreversibly to your haemoglobin
- it reduces the capacity of your blood to carry oxygen
- person dies of asphyxiation
Most fuels contain sulfur.
What is the environmental impact of this?
sulfur reacts with oxygen to form sulfur dioxide
non-metal oxides form acid when in water
sulfuric acid forms- acid rain
-lakes become acidic- killing aquatic life
-acid rain leaches minerals from soil- killing trees
How can you tell that a fraction from a distillation tower still contains a mixture of hydrocarbons?
The fraction boils over a range of temperature rather than exactly at one temperature- it is not pure
Fuel are burnt in air which contains 78% nitrogen
What is the environmental impact of this?
nitrogen reacts with oxygen to form nitrous oxide
non-metal oxides form acid when in water
nitric acid is formed- acid rain
-lakes become acidic- killing aquatic life
-acid rain leaches minerals from soil- killing trees
What product of combustion is most dangerous for the environment and why?
Carbon dioxide
CO2 is a greenhouse gas which may lead to global warming
What are the products which can form from the thermal decomposition (cracking) of heptane?
C2H4 & C5H12
Must have 7 carbons
one must be an alkene and one alkane
Long (less useful) hydrocarbons are heated and passed over a hot aluminium oxide catalyst.
This breaks up the long chain molecules into a smaller alkane and alkene.
These smaller chain hydorcarbons are more useful
What is this called?
Cracking
What are the conditions for cracking?
Hot aluminium oxide catalyst
600 - 700 °C
Explain why cracking is important to the chemical industry?
Forms smaller more useful alkanes which are in higher demand
Forms alkenes which can be used to make polymers
Cracking balances supply and demand of these smaller chained alkanes and alkenes
The products of cracking long chain hydrocarbons are shorter chained alkanes and alkenes.
How could you test to show that an alkene is produced?
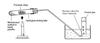
Collect gas by downward displacement
Place a few drops of bromine water
shake
should turn orange to colourless
What is the name of the polymer formed form ethene?
poly (ethene)
What is the process that links together small double bonded molecules (monomers) to form a long chain molecules.
addition polymerisation
What is the name of the polymer formed form propene?
poly (propene)
What is the name of the polymer formed form chloroethene?
poly (chloroethene)
What is the name of the polymer formed form tetrafluoroethene?
poly (tetrafluoroethene)
Which type of polymerisation is shown below
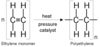
addition polymerisation
Which type of polymerisation is shown below?

Condensation polymerisation
What are the differences between addition polymerisation and condensation polymerisation?
- Addition polymers- one type of monomer
Condensation polymers - two different monomer
OR
- Addition polymerisation forms a pure polymer
Condensation polymerisation forms the polymer and a small molecule (e.g. H2O)
Describe the environmental impact of plastics and what can be done
Plastic are inert (cannot be broken down by microorganisms) AND they give off toxic gases when burned
(make them biodegradable, recycle them, reduce use, reuse them)
Name the monomer for this polymer

ethene
Name the monomer for this polymer

chloroethene
Name the monomer for this polymer

tetrafluoroethene
Name the monomer for this polymer

propene
Is this hydrocarbon saturated or unsaturated?

Unsaturated
Contains a C=C double bond
What homologous series does this hydrocarbon belong to?
What is its general formula?

alkenes
CnH2n
What homologous series does this hydrocarbon belong to?
What is its empirical formula?
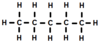
alkanes
C5H12
empirical formula is simplest ratio
5:12 is already the simplest ratio
Cracking is a thermal decomposition reaction.
True or false?
true
Bromine water added to an alkene is an example of an addition reaction?
True or false?
True
double bond opens and two bromine atoms are added to either side of the double bond
Monomers used to make addition polymers are said to be saturated?
True or false?
False
Monomers are alkenes and contain double bonds
They are unsaturated hydrocarbons
What is the main use of polyethene?
plastic bags and bottles
What is a main use of polypropene
crates and buckets
What are the main uses of polychloroethene?
window frames, plastic pipes
What is the main use of tetrafluoroethene
non-stick coating on pans
Name this organic compound

but-1- ene
- longest C chain is 4 Cs*
- double bond after 1st C*
Name this organic molecule
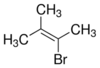
2 bromo 3 methyl but-2-ene
- Br on 2nd C*
- CH3 - methyl on 3rd C*
- double bond after 2nd C*
Name this organic compound
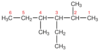
2,4 dimethyl 3 ethyl hexane
- longest chain 6 Cs long*
- methyl group on 2nd and 4th C*
- ethyl group on 3rd C*
What is a fuel?
A material which is burned to release energy
(via heating and radiation (light))
In incomplete combustion, a poisonous gas and solid may be formed. Name these.
carbon monoxide and soot
When methane reacts with bromine it forms bromomethane. UV light is needed to remove a hydrogen and replace it with a bromine atom.
What type of reaction is this?
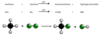
substitution

When methane reacts with bromine it needs a particular condition. Describe this condition and the resulting product.
UV light for 20 minutes
bromomethane

What do you call the hydrocarbon with a total of 8 carbon atoms in its chain with a double bond after its 3rd carbon?
oct-3-ene
What do you call the hydrocarbon with five carbon atoms in its chain and a double bond after its 1st carbon?
pent-1-ene


