§11 THERMAL PHYSICS Knowledge Flashcards
(91 cards)
What two processes can cause energy transfer between two objects.
- Heat transfer to/from object
- Work done on/by object.
What is ‘heat transfer’, ΔQ
- Heat transfer is energy transfer due to a temperature difference.
- Heat energy moves from a higher T region to a lower T region.
What is mechanical ‘work’; can you give an example of how it produces a change in U?, ΔW
- Is an energy transfer process where there is no transfer of heat, i.e. internal energy is changed by doing mechanical work,
- Work is the name given to the energy transferred when a force is applied across a distance • Work is done and there is a rise in temperature due to an increase in internal energy (remember however that no heat transfer has taken place).
What is the ‘direction’ of heat transfer under ordinary circumstances.
• Heat transfer takes place from the hotter object to the colder object.
What are the 3 types of heat transfer process?
• (1 )Radiation • (2) Convection • (3) Conduction • Can occur either to/from an object.
What is ‘internal energy’?
The internal energy of an object, is the sum of the random distribution of the kinetic and potential energies of its molecules.
How is internal energy related to the microscopic structure of a substance?
U [internal energy] is the energy of an object due to the constituent particles’: • Individual positions (potential energy) • Motion (kinetic energy)
What is ‘thermal energy’?
• Thermal energy: the internal energy of an object due to its temperature.
What’s the difference between ‘internal energy’ and ‘thermal energy’
• Internal energy U may not equal the object’s thermal energy, • ∵ Some of the U may be from sources other than thermal ones.
Can you give an example of part of an object’s internal energy not coming from a ‘thermal’ source?
• A magnetic Fe bar has greater U when magnetised then when demagnetised. • This is U but not due to an object’s temperature.
Can you give an example of work done ON an object?
• Stirring a teacup very vigorously can increase its internal energy (• This is more than offset by convection resulting in heat transfer out of the cup)
What do you know if ΔU = 0 (so U is constant)?
(1LT) ΔU = ΔQ + ΔW • If ΔU = 0, ΔQ = - ΔW • So (1) no heat transfer/work done to/from/on/by the object. OR (2) Thermal equilibrium (3) Work done on object = heat energy lost by object. (Isothermal)
What is thermal equilibrium
• No overall transfer of heat… • … between two objects of the same temperature.
Why do the brake pads of a braking car gain U?
• Moving vehicle has KE • Friction does work on BP when pressed. • KE → ΔW → U increase • Higher U manifest as higher temperature of the brake pad
How could ΔU = 0 for a filament in a running filament bulb?
• FB turned on → Elec. supply does ΔW in pushing electrons through high resistance filament. • ΔW on filament ΔU = ΔW, U increases. • ∴ Filament temperature increases to operating temp. • At O.T. negative ΔQ: heat transferred to surroundings (temp-diff) and light is radiated. • … Until ΔW = – ΔQ → ΔU = 0 • ∴Constant internal energy of lightbulb filament.
Give 4 examples of how doing mechanical work on an object changes its internal energy
• Work done by electricity e.g. on a filament bulb • banging your head on a table • hitting a golf ball with a club etc. • An important example of changing internal energy by doing work is the compression or expansion of a gas (compression - work done ON gas , expansion - work done BY gas)
What is the definition of ‘temperature’
• A measure of the average internal energy an object has. • In simple terms, ‘the degree of hotness of an object’.
What is the ‘specific latent heat of fusion’
The specific latent heat of fusion is: • the energy needed to change 1 kg of the substance from solid to liquid… • … when the solid is at its melting point (at constant temperature). • Unit of SLHF = J/kg
What’s an expression for the energy required to change (m) kg of solid to liquid at constant temperature (m.p.)
• Q = m x SPECIFIC latent heat of fusion
What is the ‘specific latent heat of vaporisation’
The specific latent heat of vaporisation is: • the energy required to turn unit mass (1 kg) of the substance from liquid state to vapour, • without causing a change of temperature (at its b.p.) Unit : J / kg - Once again, when boiling point is reached, the energy supplied does not change temperature but is used to free molecules from the liquid to become gas (or vapour).
What’s an expression for the energy required to change (m) g of gas to a liquid at constant temperature?
• Q = m x Specific latent heat of Vaporisation
When the latent heat of vaporisation is supplied to a liquid at its boiling point, where does the energy go?
• When boiling point is reached….(or when the liquid evaporates in general) • … the energy supplied does not change temperature…. • … but is used to free molecules from the liquid, to change the phase to gas (or vapour).
What are the 4 phases of matter
- Solid, 2. Liquid. 3. Gas 4. Plasma - but only at v.high temperatures (e.g. fusion reactors or stars) so not in spec.
What’s the difference between the terms ‘state of matter’ and ‘phase of matter’?
- Phase of matter ≠ state of matter necessarily • Phase : Solid / liquid / gas
- State of a system can be changed by changing temperature, pressure or volume
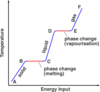
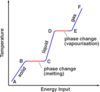
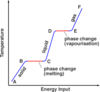
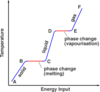
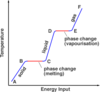
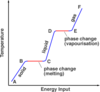
- Intermolecular forces able to pull molecules together to form a liquid.


