03 Crystallography, Structure of Crystalline Solids Flashcards
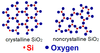
- Give a definition of crystalline solid.
a crystalline material is one in which the atoms are situated in a repeating or periodic array over large atomic distances
- Describe the difference in atomic/molecular structure between crystalline and noncrystalline materials.
atoms in crystalline solids are positioned in orderly and repeated patterns that are in contrast to the random and disorderd atomic distribution found in noncrystalline (=amorphous) materials
- Give a brief definition of a unit cell.
Crystalline solids consist of small groups of atoms which form a repetitive pattern. In describing crystal structures, is is often convenient to subdivide the structure into small repeat entities called unit cells. Most unit cells are parallelepipeds (Spat) with three sets of parallel faces.
- Draw unit cells for face-centered cubic, body-centered cubic, and hexagonal close-packed crystal structures.
A…body-centered cubic BCC e.g. Cr, Fe (α), tungsten W
B…face-centered cubic FCC e.g. Al, Cu, Au, Ag, Pb,
stacking sequence: ABCABC…
C…hexagonal close-packed structure HCP e.g. Cd, Co, Ti(α), Zn
stacking sequence: ABAB…

- Derive the relationships between unit cell edge length and atomic radius for face-centered cubic and body-centered cubic crystal structures.

- Given the atomic radius of an atom which forms into a face-centered cubic crystal structure as well as the metal’s atomic weight, compute its density.
In order to get the density we nee the volume of the unit cell. Given the atomic radius R for an FCC structure, the unit cell edge length becomes a = 2*sqrt(2)*R. Hence we get V = a³. Mass of Atoms in the unit cell is calculated as shown below:
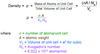
- Given the atomic radius of an atom which forms into a body-centered cubic crystal structure as well as the metal’s atomic weight, compute its density.
a = 4*R*1/sqrt(3), V=a³
Not that atomic weight A = [amu/atom(molecule) = g/mol]
1 amu = 1/12 of C12 - isotope
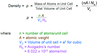
- (a) Explain what is meant by coordination number and atomic packing factor.
Two important features of a crystal structure are:
- Coordination number: the number of nearest-neighbor or touching atoms
- Atomic packing factor (APF): the fraction of solid-sphere volume in the unit cell

- (b) Cite the atomic packing factors and coordination numbers for body-centered cubic, face-centered cubic, and hexagonal close-packed crystal structures.
- BCC: APF = 0.68, Coord# = 8, 2 atoms/unit cell
- FCC: APF = 0.74, Coord# = 12, 4 atoms/unit cell
- HCP: APF = 0.74, Coord# = 12, 6 atoms/unit cell
- simple cubic: APF = 0.52, Coord# = 6, 1 atom/unit cell
- Briefly define polymorphism (or allotropy).
Polymorphism occurs when a specific material can have more than one crystal structure. Allotropy is polymorphism for elemental solids.
- Distinguish between crystal system and crystal structure.
the concept of crystal system is used to classify crystal structures on the basis of unit cell geometry - that is unit cell edge lengths and interaxial angles. There are 7 crystal systems:
- cubic
- tetragonal
- hexagonal
- orthorhombic
- rhombohedral (trigonal)
- monoclinic
- triclinic
- Recognize and also give the lattice parameter relationships for all seven crystal systems–i.e., cubic, hexagonal, tetragonal, rhombohedral, orthorhombic, monoclinic, and triclinic.

- Given a unit cell and three point coordinates, locate the point represented by these indices within the unit cell.
done by point coordinates q, r, and s
q in a-direction (x-axis)
r in b-direction (y-axis)
s in c-direction (z-axis)

- Given the location of a point within a unit cell, specify its point coordinates.
- Lattice position/coordinates are known in a metric scale: a, b, and c (e.g.[nm])
- Divide by unit cell edge lengths a, b, c and remove commas to obtain the point coordinates.
e. g. a = 0.12nm, b = 0.46nm, c = 0.20nm and
* a* = 0.48nm, b = 0.46nm, and c = 0.40nm we get:
point coordinates: 1/4 1 1/2
- Given three index integers, sketch the direction corresponding to these indices within a unit cell (for all crystal systems).

- Given a direction that has been drawn referenced to a unit cell (for all crystal systems), specify its direction indices.

- Given a unit cell and the Miller indices for a plane, draw the plane represented by these indices referenced to this unit cell.
Miller indices (hkl) are refered to all crystal systems except the hexagonal one.
reciprocal terms (n is an appropriate factor):
h = n* a/A
k = n* b/B
l = n* c/C

- Specify the Miller indices for a plane that has been drawn within a unit cell.

- Given the unit cell for some crystal structure, be able to draw the atomic/ionic packing arrangement for a specific crystallographic plane.

- Define both linear and planar atomic densities.
linear:
number of atoms centered on the direction vector divided by the length of the dircetion vector
planar:
number of atoms centered on a plane divided by the area of the plane
- For a given crystal structure, be able to determine the linear density for a specified crystallographic direction.
- For a given crystal structure, be able to determine the planar density for a specified crystallographic plane.
- (a) Draw the packing of a close-packed plane of spheres (atoms).
?
(b) Describe how both hexagonal close-packed and face-centered cubic crystal structures may be generated by the stacking of close-packed planes of atoms.
Do the same for the sodium chloride crystal structure in terms of closed packed planes of anions.
FCC and HCP crystal structures can be generated by the stacking of closed packed planes of atoms on top of each other. With this scheme A, B, and C denote possible atom positions on a close packed plane.
Stacking sequence for HCP is ABABAB…
Stacking sequence for FCC is ABCABCABC…
The same happens in some ceramic crystal structures (e.g. NaCl) by stacking of close-packed planes of anions; cations fill interstitial tetrahedral and/or octahedral positiions that exits between adjacent planes.
(c) Cite which planes in both hexagonal close-packed and face-centered cubic structures are close-packed.
close packed planes for FCC and HCP are {111} and {0001}, respectively
Remark:
Family of directions –all directions that are crystallographically equivalent (have the same atomic spacing) –indicated by indices in angle brackets
e.g. <100> = [100],[010],[001],[-100],[0-10],[00-1]
Family of planes –all planes that are crystallographically equivalent (have the same atomic packing) –indicated by indices in braces
e.g. {100} = (100), (010), (001), (-100), (0-10),(00-1)
- Distinguish between single crystals and polycrystalline materials.
single crystals are materials in which the atomic order extends uninterrupted over the entirety of the specimen (which can lead to flat faces and regular geometric shapes)
The vast majority of crystalline solids are polycrystalline i.e. they´re composed of many small crystals or grains having different crystallographic orientations.
- Define grain boundary.
is the boundary region separating two grains where there is some atomic mismatch
- Define isotropy and anisotropy with respect to material properties.
Anisotropy is the directionality dependence of properties. For isotropic materials, properties are independent of the direction of measurement.
- Briefly describe the phenomenon of diffraction.
diffraction = constructive interference
Two waves that mutually reinforce (=constructively interfer with) on another is a manifestation of diffracton, and we refer to a diffracted beam as one composed of a large number of scattered waves that mutualy reinforce on another.
- Given the angle at which an x-ray diffraction peak occurs, as well as the x-ray wavelength and order of reflection, compute the interplanar spacing for the crystallographic planes that are responsible for the diffraction peak.
see Exercise 2 - Problem 5

- For crystals having cubic symmetry, given the lattice parameter (i.e., unit cell edge length), compute the interplanar spacing for a set of crystallographic planes of specified Miller indices.
interplanar spacing dhkl
unit cell edge length a



