Week 1 - Introduction to Medical Biochemistry Flashcards
Xenobiotics
are foreign compounds the body must degrade or excrete before they accumulate or cause damage
Metabolites
typically small molecules that are intermediates in biochemical pathways or act as regulators of function.
glucose

Fructose

galactose

d-mannose

d-galactose (fisher projection)

sucrose
Glucose and fructose linked in an alpha(1-2) linkage. Table sugar, made from sugar cane or beets. sucrose is not a reducing sugar.

Lactose
Galactose and Glucose in a Beta (1-4) linkage. Milk sugar, digested by lactase in the gut in infants.

maltose
Two Glucose molecules in an alpha(1-4) linkage, the same linkage found in glycogen.
Results from starch/glycogen breakdown in the gut

maltodextrin
3 or more linearly joined glucose units in alpha(1-4) linkage
glycogen
Glucose storage in animals. Linear α(1→4) glucose chains, plus branches from by α(1→6) glycosidic bonds.
Glycogen differs from amylopectin in having more frequent branchings. This means more free ends, and a more hydrated dendrimer.

amylose
a linear, non-branched chain of glucose molecules connected by α(1→4) glycosidic bonds. Much less digestible (resistant starch) than amylopectin.
Amylose forms more compact, less hydrated structures, and is digested much slower (fewer end points, and the more compact, less hydrated structure makes it less accessible to digestive enzymes.

amylopectin
Glucose storage in plants that animals can digest. α(1→4) glycosidic bonds
with branch points of (1→6) glycosidic bond

cellulose
this is a dietary fiber. It is not digested to a significant extent in humans because we lack an enzyme to break the β(1-4) glycosidic bond, as do other mammals. Ruminants and beavers rely on gut micro-organisms to break down the cellulose into glucose as an energy source.
Blood antigens (composition)
Carb trees attached to lipids or proteins. The precise linkages and order, depends on individual proteins present and can vary among individuals. Therefore, recognition of carbohydrates as antigens can be an important aspect of immune recognition as foreign or self.
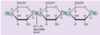
Chondroitin-sulfate
The Chondroitin-sulfate repeat provides a lot of negative charge to the sugar chains that
keeps them hydrated, and apart. This provides the elasticity required in connective tissue.
Charged Amino Acids - Anionic
Glu,Asp
Charged Amino Acids - Cationic
Arg,Lys,His
Polar Amino Acids
Ser,Thr,Gln,Asn,Cys
Non-polar Amino Acids
Leu,Ile,Met,Val,
Phe,Tyr,Trp(Aromatic)
Small Amino Acids
Ala,Glycine
Cyclic Amino Acids
Pro
primary structure protein
Proteins are typically described as N-terminus (free amino terminus) to C-terminal. Amino acids are conjoined through peptide bonds. Peptide bonds are amide bonds (bonds between a carboxylate group and an amine). The sequence of amino acids conjoined through peptide bonds.





