Week 1 Flashcards
(203 cards)
1
Q
Function of the immune system
A
To defend against invasion of the body by pathogens
2
Q
What are the two types of pathogens?
A
- Those that will cause disease in everyone 2. Those that will only cause disease in immune-compromised individuals (opportunistic pathogens)
3
Q
What’s the first line of defense in the immune system?
A
- Non-specific barriers include:
- Skin
- Mucus of exposed areas (respiratory tract, vagina, etc.)
- Acidity of stomach - prevents bacterial growth
- Lysosyme of tears and saliva - enzyme tha tkills bacteria by destroying cell wall
- Defensins - secreted by the immune system, inhibit bacterial growth
4
Q
What’s the 2nd line of defense against pathogens?
A
Innate immune system
5
Q
Features of the innate immune response
A
- Acquired
- Not specific to invading pathogen
- Always results in inflammation
- including complement activation, phagocyte destruction of microorganisms, cytokine production and Natural Killer cell activity
6
Q
What’s the third line of defense?
A
Adaptive immune system
7
Q
Features of the adaptive immune system
A
- Specific to invading pathogen
- Involves lymphocytes
- Receptors are specific to invading pathogens, unlike innate immunity
- These receptors are distributed on lymphocytes
8
Q
members of the same clone
A
- refers to lymphocytes that have receptors for the same pathogen
9
Q
clonal expansion
A
- when a lymphocyte recognizes a pathogen, that lymphocyte divides and generates a lot more lymphocytes with the same receptor for the invading pathogen

10
Q
adaptive immune response
A
- pathogen recognition
- clonal expansion

11
Q
memory cells
A
- after a pathogen invades, some lymphocytes undergo clonal selection to respond to the pathogen
- Other lymphocytes develop into memory cells to respond very quickly should the same pathogen invade again
12
Q
timing of innate vs adaptive immune system
A
- innate response is immediate. It basically keeps the pathogens in check while adaptive response ramps up
- adaptive response is a little slower
13
Q
leukocytes
A
- white blood cells
- include lymphoid and myeloid lines (B-cell, T-cell, natural killer, neutrophil, eosinophil, basophil, monocyte, dendritic cell, macrophage)
14
Q
Granulocytes
A
- Neutrophil
- Basophil
- Eosinophil
- “Polymorphonuclear leukocytes” due to shape of nuclei
- Granular cytoplasm for destruction of pathogens
- All are phagocytic
15
Q
neutrophil
A
- Type of granulocyte, derived from myeloid line
- Most abundant leukocyte
- Innate immune response
- Bind C3b –> phagocytosis in complement system
- C5a released during complement system is strong chemoattractant for neutrophils
- Interleukins released after toll-like receptors bind ligand –> bone marrow increases neutrophil production
16
Q
basophils and eosinophils
A
- Granulocytes
- Derived from myeloid precursor
- Immunity to parasites
17
Q
Monocytes
A
- Myeloid line
- differentiate into macrophages
- Phagocytic
- Live for years whereas neutrophils only live for 24-48 hours
- Largely in innate immune response
18
Q
dendritic cells
A
- phagocytic
- Work in adaptive immune response
- Carry pathogen fragments from site of infection to secondary lymphoid organs
- Present antigen fragments to T-cells
19
Q
Natural killer cells
A
- Innate immune response
- Activated by IL-12, IFN-B, TNF-alpha (which are all results of TLR binding its ligand)
- Central to viral immune response
20
Q
lymphocytes
A
- B-cells
- T-cells
- Adaptive immune response
21
Q
T-cells
A
- Mature in the thymus
- adaptive immune response
- lymphocyte
- Receptor: “T-cell antigen receptor”
22
Q
B-cells
A
- Cell surface receptors: antibodies
23
Q
Primary lymphoid organs
A
- bone marrow, thymus
24
Q
secondary lymphoid organs
A
- spleen, appendix, tonsils, adenoid, lymph node, Peyer’s patches
25
where does the intitial lymphocyte recognition of pathogens take place?
* Secondary lymphoid organs
26
Describe the process of lymphocyte recirculation
* lymphocytes do not stay in a single tissue forever. They go to the tissue and hang out, and if there is no pathogen that they are specific to, that lymphocyte will leave and go back into blood circulation
* lymphocyte can be sent to a different tissue to hang out there and wait for its pathogen
27
how do pathogens get to the lymph nodes, where they can be recognized by lymphocytes?
* afferent lymphatic vessels
* Can be brought in by macrophages/dendritic cells or as free antigen
28
where in the lymph node are T cells found?
* paracortex (inner cortex)
29
where in the lymph node are B-cells located?
* outer cortex
30
germinal center
* area of lymph node with lots of b-cell activity
31
Overview of adaptive response in lymph node
* At site of infection: macrophages and dendritic cells chew up pathogen. Macrophages and dendritic cells carry fragments of pathogen to the lymph node. Pathogen also travels to lymph node as free fragments.
* Dendritic cells (with pathogen fragments) go to inner cortex of lymph node where T-cells are located. Dendritic cells trigger T-cells to start differentiating specific to that pathogen.
* Loose pathogen fragments travel to lymph node and bind to B-cells, triggering B-cells to proliferate.
* Activated T-cells help B-cells defferentiate into plasma cells
* \*\*B cells have antibodies on their cell surface; plasma cells SECRETE antibodies\*\*
* Some T-cells went to lymph node, others leave lymph node and go directly to site of infection
32
Adaptive response in spleen
* Pathogens gain access directly from blood as opposed to via afferent vessels (lymph node)
* Lymphocytes differentiate same as they do in lymph node
* But then lymphocytes go directly into blood from the spleen, unlike lymph node process (which utilizes efferent vessels)
33
MALT
* mucosa-associated lymphoid tissue
* This includes the other secondary organs: tonsils, adenoids, appendix, peyer's patches
* T-cells and B-cells occupy different areas in these organs, just like in lymph node and spleen
* Distinguishing feature of MALT: pathogens enter through **M-cells**, which are specialized epithelial cells that transport pathogens in
34
drug absorption
* getting drug from site of administration into the blood
35
drug distrubution
* getting drug from the blood to tissues
36
What are the fates of free drug after it has been absorbed?
1. Storage in tissue (i.e. fat)
2. Drug metabolism (happens in the liver) -- then excreted usually
3. Excretion
4. Site of action
37
what is the first pass effect
* This is the metabolism of drugs in the liver before they can get into the bloodstream
* The drug gets ingested orally, and is taken from the small intestine directly to the liver before reaching the blood
38
Mechanisms of drug transport across membranes
1. Diffusion
2. Active transport
More that we didn't focus on
39
What must be true of a drug in order for it to diffuse across a membrane?
* It must be lipophilic enough - must NOT be ionized.
40
One main difference between diffusion and active transport as far as drug distribution is concerned
* active transport is a saturable process, whereas difficusion is not
41
What are the main chemical properties that affet drug transport?
1. Lipid Solubility
2. Acid/base properties
42
How do we measure the lipid solubility of a drug?
* Lipid-water partition coefficient
* Amount of drug in organic phase/amount of drug in aqueous phase
* Higher coefficient = more drug in organic phase = more lipophilic drug
43
What does lipid partition coefficient tell you?
* Roughly correlates with drug absorption
* The higher lipid-water partition coefficient, the more lipophilic the drug, the better it is at diffusing across membranes
44
How do acid-base properties of a drug affect its absorption?
* Drugs cannot cross membranes in their ionized form
* This will impact drug getting into bloodstream (absorption) and drug getting into different tissues (distribution)
45
What is ion trapping?
* Different body compartments have different pH's
* A drug may be unionized in a certain compartment and able to cross membranes, but then enter a different compartment and become ionized
* When it becomes ionized, it is "trapped" in that body compartment
46
common example of ion trapping
* Amphetamine is a weak base
* If someone is having an overdose, you can acidify the urine. This adds hydrogens to the urine --\> amphetamine accepts hydrogens --\> amphetamine becomes ionized and is "trapped" in the urine
* Now the amphetamine can be excreted
47
another example of ion trapping (phenobarbitol)
* This is a weak acid
* If you acidify the blood, the blood will be proton-rich. The drug will accept protons and become UNionized. (O- --\> OH)
* Drug levels in the blood will DECREASE b/c the drug can now be distributed to the tissues
* Result: more of the drug can reach the brain, which deepens anesthesia
48
how to lighten anesthesia with phenobarbitol example
* Drug is a weak acid
* You can deepen anesthesia by acidifying blood --\> more of the drug is in unionized form --\> more drug gets absorbed into brain
* When you make blood more basic, the unionized form is found in the blood. There's an equilibrium between ionized and unionized form that gets shifted to the ionized form in this case.
* When there's less of the unionized form in the blood, the unionized form of drug in the brain will diffuse out down its concentration gradient.
* Once in the blood, the drug will become ionized and not diffuse back into tissue.

49
What's a liability of a low-absorption blood?
* GI tract has massive surface area for absorption
* If a drug doesn't typically get absorbed well, you can just give a huge dose. B/c of the GI tract surface area, some fraction of it will get absorbed.
* Problem is some patients may absorpt more than others. If one patient absorbs 1% and another 10%, it could be toxic to the 10% patient.
50
Active transport
* Many drugs interact with transporters instead of diffusing across cell membranes
* The competition for these transporters can be the basis of drug-drug interactions
51
P glycoprotein
* This is a transporter that pumps drug out of the cell -- i.e. it is an "efflux" transporter
* It is driven by ATP

52
p glycoprotein inhibitors
grapefruit juice
53
p glycoprotein inducers
* St. John's wort
* An inducer makes p glycoprotein work better, which means more of the drug is pumped back OUT of the epithelial cells into the small intestine and less is absorbed into the blood
54
What determines how much drug is absorbed in the case of active transport?
1. Drug dose
2. Drug efflux - i.e. How much of the drug is pumped back out of the epithelial cells into the intestine by P-glycoprotein
3. Inhibitors/inducers of P-glycoprotein
4. Competition for the transporter
5. metabolism of drug within epithelial cells
55
How can grapefruit juice set up drug overdose?
* Grapefruit juice is an inhibitor of p-glycoprotein, which is a pump that actively pumps drug back into small intestine
* when grapefruit juice is present, the p-glycoprotein can't pump as much back into small intestine --\> more of the drug gets absorbed
56
role of albumin in drug absorption
* Some drugs bind albumin in the blood
* When the drug is bound to albumin, it is not active
* With so much bound drug in the blood, you can get drug-drug interactions.
57
what are the mechanisms of innate immunity?
1. complement system activation
2. phagocytosis
3. inflammation
4. toll-like receptors
5. innate interferon response
6. natural killer cell activity
58
what type of pathogen activates the complement system?
All pathogens
59
what activates phagocytosis?
all pathogens
60
what activates inflammation
all pathogens
61
what activates toll like receptors?
all pathogens
62
what activates the innate interferon response?
viruses only

63
what activates natural killer cell activity
viruses only
64
who are the key players in phagocytosis?
* macrophages
* inflammatory neutrophils
* inflammatory monocytes
65
where do the key players of phagocytosis reside?
* macrophages - reside in tissue AND blood
* neutrophils - only blood
* monocytes - only blood
66
what is the role of C3B in phagocytosis?
* Gets bound to the pathogen
* Allows phagocytes to recognize the pathogen
* Phagocytes have a receptor (CR1) for C3B, making it easier to ingest the pathogen
67
how does phagocytosis lead to pathogen death?
* phagocytes ingest pathogen and then fuse with lysosome --\> phagolysosome
* Enzymes in phagolysosome chew up pathogen
* Reactive oxygen species in phagolysosome digest pathogen
* Nitric oxide fucks up the pathogen (how?)
68
what enzymes are found in the phagolysosome?
* collagenases
* elastases
* hydrolases
69
what is opsonization?
* the process of marking pathogens with C3b to make them easier to identify by phagocytes
* Makes them easier to phagocytose
70
Which complement pathways are involved in the innate immune response?
* All 3
* Classical
* Alternative
* Lectin
71
which complement pathway(s) are involved in adaptive immune response?
* only the classical pathway
72
Overview of complement pathways

73
what is complement fixation?
* The binding of C3b to the pathogen covalently

74
what are the main chemoattractants of the complement pathway?
* C3a
* C5a
75
NADPH oxidase
* Functions in generating reactive oxygen species in phagosomes
* These ROS's help destroy pathogens
76
membrane attack complex
* Formed as part of the complement system
* Pokes holes in the pathogen membrane leading to lysis
77
what are the 4 characteristics of inflammation?
heat
swelling
redness
pain
78
how do C3a and C5a result in inflammation?
* They're generated at the site of injury
* Diffuse away and interact with epithelium of blood vessels in the region of the injury
* They cause the blood vessel to increase permeability so that more macrophages and neutrophils can come in to the damaged tissue from the blood
* This also causes debris from site of injury to be carried away (extravasation)
79
How does inflammation promote innate immunity?
* Allows phagocytes to come in to site of tissue damage
* Allows pathogen debris to leave site of injury
80
what's the role of toll-like receptors?
* function in innate immune response
* They are receptors that recognize certain things on a pathogen
* They activate the phagocyte to synthesize and release cytokines that will recruit more immune cells to the site of tissue damage
81
what are the 2 most important toll-like receptors?
* toll-like receptor 3 (TLR 3)
* toll-like receptor 4 (TLR 4)
82
what are the ligands for TLR 3?
double stranded viral RNA
83
what are the ligands for TLR4?
lipopolysaccharide
84
what kinds of microorganisms does TLR 3 recognize?
viruses - e.g. West Nile Virus
85
what kinds of microorganisms does TLR 4 recognize?
gram negative bacteria
86
what kinds of cells carry TLR 3?
Natural killer cells
87
what kinds of cells carry TLR 4?
macrophages
dendritic cells
mast cells
eosinophils
88
where is TLR 3 found on/in the cell?
endosomes
89
where is TLR 4 found on/in the cell?
plasma membrane
90
TLR features
1. Each TLR recognizes a feature that is common to a number of different of pathogens
2. TLR's recognize enough features that they can basically cover the entire microbial world
3. TLRs are expressed at sites within cells that their ligands are likely to be encountered
Ex: TLR 4 recognizes lipopolysaccharide, which will be on the surface of a gram negative bacteria. So TLR 4 is found on the plasma membrane of the cell.
Ex: many viruses use endocytosis to gain access to a cell. Since TLR 3 recognizes viral RNA, TLR 3 is found in the endosome
91
what cytokines are released by TLR recognition of pathogens?
1. IL-12
2. CXCL 8
3. TNF-alpha
4. IL1-Beta
5. IL-6

92
what is unique about CXCL8?
* It's a chemokyne rather than a cytokine - a cytokine with chemoattractant properties
93
function of CXCL8
* released from macrophage when TLR binds its target
* recruits neutrophils to site of infection
* It's a chemokyne
94
function of IL-12
activates NK cells
95
neutrophils
* phagocytes of innate immune system
* recruited to site of infection by C3a, C5a, and CXCL8
* express many of the same receptors on cell surface as macrophages, EXCEPT for TLR's
* shorter lived than macrophages
96
TNF-alpha
* released from macrophages when TLR binds pathogen
* increases vascular permeability and recruits more immune cells to site of infection
97
IL1-Beta
* Released from macrophages when TLR binds pathogen ligand
* Increases vascular permeability and recruits immune cells to site of infection
98
what are the systemic effects of cytokine release? Which cytokines are responsible?
* IL-1-beta
* IL-6
* TNF-alpha
* released from macrophages when TLR binds ligand
* Produce a fever by: 1. increasing metabolism 2. acting on hypothalamus to increase body temperature
* trigger neutrophil release from bone marrow
* IL 6 specifically triggers the release of acute phase proteins
99
what triggers the acute phase response?
* IL-6
100
what is the acute phase response?
* liver releases acute phase proteins:
* Mannose-binding lectin
* C-reactive protein
101
mannose-binding lectin
* triggers activation of the lectin pathway when it binds mannose on pathogen cell surface
* released by liver in response to IL-6, which itself is released from macrophages when TLR recognizes its ligand on a pathogen
* acute phase protein
102
c-reactive protein
* activates the classical pathway
* released from liver as response to IL-6, which itself is released from macrophages when TLR binds its ligand on pathogen
* acute phase protein
103
What is the innate response to viral pathogens?
1. Interferon release
2. NK cell response
104
Type 1 interferons
* IFN-alpha
* IFN-beta
* act as cytokines
* These are the innate interferons
105
Type 2 interferons
* IFN - gamma
* "Immune interferon"
* More important in adaptive immunity
106
what does IFN-beta do?
1. triggers release of IFN-alpha
2. binds to own cell or neighboring cell --\> interferon response
Interferon response = cell inhibits further viral replication
107
what is the interferon response?
* triggered by binding of IFN-alpha or IFN-beta
* when these interferons bind, the cell:
* changes its protein expression to inhibit further viral replication in that cell
* becomes more susceptible to attack by NK cells
* interferons bind to NK cells to make them more active
* \*\*\*Innate interferon response = inhibition of further viral replication\*\*\*
108
How are NK cells activated?
* Type 1 interferons (INF-alpha and beta)
* IL-12
* TNF-alpha
109
what do NK cells do?
* part of innate immune response
* control infection until adaptive immune response can kick in
110
role of IL-12 with natural killer cells
* recruits NK cells to site of infection
* acts on NK cells to increase killing potential
* Triggers NK cells to secrete type 2 interferon (INF-gamma)
111
INF-gamma
* Released by NK cells during immune response
* Role is in adaptive immune response
112
Types of NK cell receptors
1. immunoglobulins (bind antibodies)
2. lectin-like (bind carbohydrates)
--Within each of these groups of receptors, there are inhibitory and activating receptors
--Activating receptors trigger NK cell activation --\> killing. Inhibitory receptors do the opposite.
--In a healthy cell, ligands are expressed that are inhibitory signals to NK cells --\> inhibit NK cells from killing.
113
The two main changes to drugs during drug metabolism
1. Making the drug more polar
2. Conjugation
114
Phase 1 enzymes in drug metabolism
* Introduce a polar functional group
* (-OH, -NH2, -SH, or -COOH)
115
Phase 2 enzymes in drug metabolism
* conjugate something onto the drug
116
types of conjugation in drug metabolism
* glucuronide
* sulfate
* glutathione
* amino acids
117
cytochrome p450
* the main enzymes used in phase 1 drug metabolism
118
what kinds of reactions do cytochrome p450 catalyze?
redox
119
where are p450 enzymes found?
endoplasmic reticulum membrane
120
mechanism of p450 enzymes
* Use heme and NADPH
* Heme is the site of oxygen activation and catalysis
* catalyze redox reactions
*
121
what are the most prevalent p450 enzymes?
* CYP1, CYP2, CYP3
* CYP3A is the single most active of the p450 enzymes
122
What does St. John's wort do to CYP3A?
* It's an inducer
* If you treat a patient with cyclosporin and start giving St. John's wort, you will increase metabolism of cyclosporin and it can fall below therapeutic levels
123
What does St. John's wort do to p-glycoprotein?
an inducer
124
St. John's wort relationship to drug metabolism
* It's an inducer of both CYP3A and p-glycoprotein
* St. John's wort --\> drugs are metabolized faster by CYP3A AND drugs are not absorbed b/c p-glycoprotein is more active --\> less drug is absorbed
125
what does grapefruit juice do the bioavailability of felodapine?
* grapefruit juice is an inhibitor of p-glycoprotein and p450 enzymes
* felodapine is metabolized by p450 enzymes
* Grapefruit juice results in less metabolism of felodapine and less efflux (p-glycoprotein ships it back into small intestine)
* Result: much more felodapine in the blood
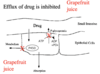
126
Flavin Monoxygenase (FMO)
* Another enzyme family important in drug metabolism
* Distinct from p450 enzymes
127
What kinds of reactions do FMO enzymes catalyze?
redox (like p450)
128
what is the prosthetic group for FMO enzymes?
FAD
129
whats the cofactor for FMO enzymes
NADPH
130
whats the difference b/w FMO and p450 enzymes?
* p450 use heme as a cofactor
* FMO use FAD as cofactor
131
trimethylamine
* responsible for the fish body odor smell
132
name 2 reactions catalyzed by FMO
1. nicotine --\> nicotone-1-N-oxide
2. trimethylamine --\> trimethylamine-N-oxide
133
what is the cause of the fish body odor smell?
* Mutation in FMO --\> less metabolism of trimethylamine
* build up of trimethylamine causes the fishy body odor smell
134
microsomal vs. non-microsomal enzymes
* microsomal enzymes are found in the endoplasmic reticulum and typically catalyze reactions involving lipophilic substrates
* non-microsomal enzymes are found in places other than the ER
135
Name 2 examples of non-microsomal enzymes used during drug metabolism
1. alcohol dehydrogenase
2. aldehyde dehydrogenase
136
What is the overall process of ethanol metabolism?

137
what is the cause of the "asian flush" (when drinking alcohol)
* Mutations in alcohol dehydrogenase and acetaldehyde dehydrogenase
* results in Alcohol dehydrogenase metabolizes ethanol FASTER
* results in Acetaldehyde dehydrogenase metabolizing its substrate - acetaldehyde - slower
* Buildup of acetaldehyde causes the redness
138
what is significant about the metabolism of methanol and ethylene glycol?
* the products of their metabolism are toxic
139
what are the metabolites of methanol
formaldehyde
formic acid
140
how can you manage toxicity of methanol and ethylene glycol?
* provide a drug (4MP) that blocks their metabolism
* give lots of ethanol, which is also a substrate for the enzyme used to metabolize methanol and ethylene glycol. Ethanol will crowd out binding and metabolism of the other two.
141
overall process of phase 2 enzymatic reactions

142
glucuronidation
* phase 2 drug metabolism (conjugation)
143
what is the main enzyme used in glucuronidation?
UDP-glucuronosyl transferase
144
what is the cofactor for UDP-glucuronosyl transferase?
UDP-glucuronic acid
145
what is the mechanism of glucuronidation?
* Attach glucuronic acid to the drug
* why? --\> attaching a polar group makes the drug easier to excrete

146
Cause of Grigler-Najjar syndrome and Gilbert's disease
* mutation in UDP-glucuronosyltransferase
* This enzyme is involved in glucuronidation, but it's also involved in the metabolism of billirubin during heme breakdown
* Mutation --\> build up of billirubin --\> jaundice
147
why do newborns come out jaundiced?
* The glucuronidation pathway isn't working yet
* This pathway is responsible for the metabolism of billirubin
* Not enough glucuronidation --\> too much billirubin --\> jaundice
148
Acetylation
* phase 2 metabolism (conjugation)
149
enzyme used in acetylation pathway
N-acetyltransferase
150
cofactor for acetylation pathway of drug metabolism
acetyl-CoA
151
overall reaction of acetylation in drug metabolism

152
glutathione conjugation
phase 2 metabolism
153
what is glutathione?
* Made up of 3 amino acids: glutamic acid, cysteine, and glycine
154
what enzyme is used in glutathione conjugation?
glutathione S-transferase
155
what is the cofactor in glutathione conjugation?
glutathione
156
what is the signature molecule for the glutathione pathway?
mercapturic acid
157
sulfation
phase 2 drug metabolism
158
cofactor in sulfation pathway of drug metabolism
PAPS
159
cofactor in methylation pathway of drug metabolism
S-adenosylmethionine (SAM)
160
2E1
* A p450 enzyme responsible for metabolizing acetaminophen
161
what is the basis of the interaction between acetaminophen and alcohol?
* ethanol upregulates 2E1 activity (it's an inducer of 2E1)
* At low doses of acetaminophen, it is metabolized via glucuronidation or sulfation
* At higher doses of acetaminophen, 2E1 metabolizes it and produces a reactive intermediate. This reactive intermediate can be metabolized either to a non-toxic substance via glutathione pathway OR results in liver toxicity
* IF 2E1 has been upregulated by ethanol consumption, you get more of the reactive intermediate, you can overwhelm the glutathione pathway, and cause liver failure.

162
factors that affect drug metabolism
* Age
* Ex: jaundice at birth b/c glucuronidation pathway isn't up and running yet
* Ex: Diazepam has a 20-hour half life in a young person but an 80 half life in the elderly
* Species
* Cats cannot do glucuronidation
* Genetics
* Ex: Mutations in japanese population for ethanol metabolism
163
prodrugs
* Activated by drug metabolism
164
halothane metabolism
* There are 2 pathways: reductive and oxidative
* The oxidative pathway forms a reactive intermediate that covalently alters liver proteins
* In a very small number of people, the immune system is reactive to these covalently altered liver proteins, which results in liver failure
165
what are the 3 complement pathways?
1. classical
2. lectin
3. alternative
166
how is the classical pathway initiated?
* antibody binds an antigen OR
* C-reactive protein binds antigen
167
how is the lectin pathway initiated?
* mannose binding protein binds to a pathogen surface
168
how is the alternative pathway initiated?
* auto activation of a complement component on the surfae of the pathogen
169
what is the result of initial binding for all 3 pathways?
* C3b binds to the pathogen surface
170
what are the 3 options that result from C3b binding?
1. opsonization
2. inflammation
3. lysis
171
convertase
* non-covalent proteins together that function to cleave a component of complement
* Ex: C3 convertase is a group of complement proteins that cleaves C3 into C3A and C3B
172
C1q
* binds the antibody in classical pathway
173
C1r and C1s
Enzymatic activity of C1 in classical pathway
(C1q binds to the antibody)
174
what does C1s cleave?
* C4 --\> C4a and C4b
* C2 --\> C2b and C2a
175
C3 convertase of classical pathway
* C4b, 2b
176
C3 convertase action
cleaves C3 --\> C3a and C3b
177
what does C1q bind?
* A single IgM (b/c IgM is pentameric)
* Multiple IgG
178
steps of classical pathway up to C3b binding

179
what initiates the lectin pathway?
* mannose binding protein binds mannose on the pathogen surface
180
What is the C3 convertase of the lectin pathway?
* C4b, 2b
181
overview of the lectin pathway up to C3 binding

182
what initiates the alternative pathway?
* autoactivation of a C3 molecule --\> C3b binds pathogen
183
C3 convertase of alternative pathway
* C3b, Bb
* Cleaves C3 into C3b and C3a
* Each new C3b can bind Factor B--\> formation of another C3 convertase
184
C3 convertase activity of alternative pathway does what?
amplifies C3b formation for classical and lectin pathways
185
opsonization
* the process of marking a pathogen for destruction by phagocytes
186
Type 1 complement receptors (CR1)
* Found on macrophages and neutrophils
* Bind C3b
187
complement fragments that mediate inflammation
* C3a and C5a mainly
* C4a a little
188
anaphylatoxins
* C3a, C4a, C5a
* So called b/c when injected into animals, they cause anaphylaxis
* These mediate/cause inflammation
189
C5a
* mediates inflammation
* acts as a strong chemoattractant for neutrophils and monocytes to recruit them to damaged tissue
190
what is responsible for lysis of pathogens in complement system?
Membrane Attack Complex
191
How does the MAC form?
* C3b complexes with a C3 convertase
* These 3 proteins form a C5 convertase
* C5 is cleaved and C5b complexes with C3b
* C6, C7, C8, and C9 complex onto this. C7 and C8 insert into membrane

192
Deficiencies in C1, C4, and C2 lead to problems in which pathway?
classical
193
what's the result of deficiencies in classical pathway proteins?
* Can't clear immune complexes from the blood
* Results in joint problems (arthritis)
* Kidney problems (renal disease)
* skin problems (rash)
* symptoms similar to lupus
194
Deficiencies in C3, Factor B, and properdin cause problems in which pathway?
* alternative pathway
195
what is the result of deficiencies in alternative pathway?
* harder to opsonize and phagocytose
* more at risk for infection by pyogenic bacteria (bacteria that result in production of pus)
196
Deficiencies in C5, C6, C7, C8, or C9 result in what problem?
* can't form the MAC
* increased risk of Neisserial infection --\> MENINGITIS
* B/c MAC is crucial for handling gram negative bacteria, which Neisserial is
197
Regulator proteins of comlement pathway
* C1 Inhibitor (C1-INH)
* DAF (Decay Accelerating Factor)
* MCP (membrane cofactor protein)
* CR1 (Type 1 complement receptor)
* Factor I
* Factor H
* SPCN
198
C1 inhibitor
* inhibits C1 in classical pathway
* deficiency --\> HANE (hereditary angioneurotic edema)
199
Decay Accelerating Factor
* Inhibits all 3 complement pathways
* Deficiency --\> hemoglobinuria due to RBC's increased susceptibility to attack
200
Protectin (CD59)
* GPI-anchored protein on host cells
* Inhibits the formation of MAC
* deficiency --\> hemoglobinuria
201
CH50
* Patient serum has complement proteins in it. You add patient's serum in varying concentrations to RBC's and see how well the patient's blood lyses RBC's
* More lysing = complement system working better
* CH50 = concentration of patient serum at which 50% of RBC's are lysed. This is a measure of function of a patient's complement system.
* If patient A needs a higher serum concentration to lyse RBC's than patient B, patient A's complement system is less active.
202
How do pathogens get to lymph node vs. spleen vs. MALT?
* lymph node: enter via afferent vessels, leave via efferent
* spleen: enter and exit directly from the blood
* MALT: specialized epithelial cells called M-cells transport pathogen in
203
Overview of phase 2 enzymes and their cofactors



