Ventilation and gas exchange week 5 Flashcards
What are the 2 types of ventilation? How are they calculated?
MINUTE VENTILATION:
• is the volume of gas ventilated in one minute
• measures the air that enters and leaves the trachea
• shows how fast air moves in and out of the lungs (but does NOT show how much contributes to gas exchange)
• is calculated as VE = f × VT
MINUTE VENTILATION = BREATHS/MIN × TIDAL VOLUME
Only a part of the air that is inspired is available for gas exchange with the blood. The last part of each tidal inspiration stays in the anatomic dead space and therefore does not reach the alveoli, and its composition is unaltered by gas exchange with the pulmonary capillaries. (That same air is the first gas that is breathed out during expiration.) We use the term “alveolar ventilation” to refer to the gas that reaches the alveoli where it is available for gas exchange.
ALVEOLAR VENTILATION:
• is the physiologically useful part of ventilation, the “good” or effective ventilation that contributes to gas exchange
• is the volume of gas available to the alveolar surface per minute
• is the air that enters and leaves the alveoli (respiratory zone) via diffusion
• is expressed as
VA= f × (VT - VD)
ALVEOLAR VENTILATION = BREATHS/MIN × (TIDAL VOLUME–DEAD SPACE)
Reminder – dead space (VD) is where air sits in the conductive zone (trachea and bronchioles) and does not contribute to VA. A “Rule of thumb” is that the dead space volume (VD) in milliliters is roughly equal to a person’s body weight in pounds.

What are the two types of dead spaces?
Anatomic dead space
o Regions incapable of O2 and CO2 exchange with the blood
o Volume of the conducting airways
o ~150 ml in a “normal” person (VD in ml body weight in pounds)
Physiologic dead space
o Anatomic + “alveolar dead space” (alveoli that have ventilation without blood flow, therefore, no gas exchange)
o Volume of lungs that doesn’t participate in gas exchange
o In normal lungs, same as anatomic dead space
o In diseased lungs, may be larger than anatomic dead space
o Functional measurement
How is physiologic dead space calculated?
Do not memorize this! You will not need to calculate anything with the Bohr Equation on the exam. However, you DO need to understand why this equation works! This cartoon illustrates the concepts that allow the measurement of Physiologic Dead Space. Inspired air has ~0 CO2 whereas alveolar air has PA,CO2 40 torr. Therefore, if you measure the PCO2 of a mixture of air from these two sources (PE,CO2 in the equation above), you can calculate what fraction was in Physiologic Dead Space. For example, if PE,CO2 = 30, then ¼ of VT was in Physiologic Dead Space, because [40-30]/40 = 0.25. In pulmonary disease, if there is a region of the lung in which no gas exchange takes place, this will behave just like Physiologic Dead Space. If, as is more likely, there is a region where gas exchange occurs poorly, then this will increase Physiologic Dead Space, as well.

Keeping minute ventilation (VE) consant, what is the most effective way to increase alveolar ventilation?
How can increasing dead space (as occurs in some lung disease) impact VA?
The same total minute ventilation rate, VE, can be achieved by different patterns of breathing (first 3 rows).
However, VA is greater for deep, slow breathing (high VT and low f). This is because part of every breath just sits in dead space, and does not contribute to alveolar ventilation (no gas exchange occurs in anatomical/physiological dead space). If dead space is constant, taking a bigger breath (a higher VT) means more air can get into the respiratory zone.
The fourth row shows that a normal breathing pattern (like row 1) in a patient with increased physiologic dead space results in subnormal VA. Increased dead space means that the patient gets less benefit from breathing, and must work harder to achieve a normal VA.
Bottom Line: Varying f and VT may keep VE constant but does not necessarily mean that VA will stay constant.

How can work of breathing be determined? (think of the equation used to determine work, the forces the lung has to work against, and how work of the lung is plotted)
How does the work of breathing change with increasing frequency (breaths/min)?
Thw work done by respiratory muscles in breathing can be obtained from static and dynamic pressure-volume curves.
W= force × distance = ∆P × ∆V
To increase the lung volume (attached), work must be done against elastic recoil of the lung. This work can be approximated as the blue triangular area, which starts from the static compliance curve. In real life, when we breathe, the pressure and volume change continuously, which is a dynamic compliance curve (red). We still have to do the same work against elastic recoil, but now we do additional work against airway resistance. This work is shown as the red area. If we breathe faster, this requires more work against airway resistance (green area) because we are forcing the air to move faster and the curve bulges farther out.
The total work is the sum of both components of work:
• Static Compliance Curve
o Elastic work (work against elastic recoil of the lungs and chest wall)
• Dynamic Compliance Curves
o Work against airway resistance Raw

Oxygen consumption by respiratory muscles can be used to reflect the work they do. Use the attached graph to describe work done by respiratory muscles in a normal person (that is sitting at rest) to a person with emphysema. Why does a person with emphysema do more work just do breathe?

How much work do those respiratory muscles do, anyway? We breathe all of the time. Attached figure shows the rate of total body O2 consumption in resting subjects during voluntary hyperventilation. Increasing the minute ventilation rate, VE, increases the work done. Work is measured as the rate of O2 consumption by the respiratory muscles.
Normal Individual:
A normal person sitting quietly consumes ~250 ml of O2 per minute, which is the baseline for this graph. At a resting VE ~5 liters/min, the respiratory muscles consume a very small fraction (1-2%) of the total metabolic O2 demand. During vigorous exercise, VE increases enormously, and the work that it takes to sustain this high VE also increases.
Emphysema:
Now consider the poor geezer with emphysema. His lungs are so shot that it takes a tremendous amount of work just to breathe. Moderate exercise (walking up a flight of stairs) increases the work done by the respiratory muscles (i.e., their O2 consumption plotted in attached graph) to the work required by a normal person running a marathon. With strenuous exercise, this patient may use all of the O2 taken in just to power his/her respiratory muscles. Dynamic compression of airways in emphysema increases the amount of work needed just to breathe normally. This is obviously a losing strategy.

Describe the changes in work with the parameters listed in this figure. Be sure to discuss the “total” line and what this means for normal breathing.

Low frequency breathing: To breathe at low frequency requires a high VT, so the lungs have to stretch beyond the high compliance region of the P-V curve, the flatter part of the curve (remember?). This requires more work against elastic recoil.
High frequency breathing: Work against Raw increases at high f because the respiratory muscles must contract harder to force the air in and out of the lungs at a higher rate (they must generate a higher pressure gradient to make the air move faster). At high f turbulence becomes significant, which further increases work because turbulent air flow is less efficient.
Total: extreme breathing patterns are inefficient and require a lot of work. People breathe around 12 breaths/min where work is minimized
- Bottom line: Low frequency –> high VT –> more work
- Bottom line: High frequency –> high turbulence –> increased contraction –> more work
- Bottom line: Middle frequency–> minimize work
Describe the differences in the work of breathing at different frequencies for restrictive vs obstructive disease.

Restrictive disease: Pts have increased elastic recoil which makes it harder to inflate their lungs. The most efficient way for them to breathe is to increase f and reduce VT. Total work increases because higher f means greater VE is needed to achieve the same VA.
Obstructive disease: Elastic recoil of the lungs is decreased (which lowers the pressure inside airways). Airway resistance increases during expiration due to dynamic compression of airways. As discussed, increasing breathing f increases Raw. Therefore, it is more efficient for a person with obstructive disease to breathe at lower frequencies.

What is partial pressure? What is Dalton’s law of partial pressure? How is partial pressure of a gas calcuated?
Dalton’s Law states that the total pressure exerted by a mixture of gases is equal to the sum of the pressures that would be exerted by each of the gases if it alone were present and occupied the total volume. In other words, if you have a mixture of gases, each individual gas acts like the other gases are not around. The concept of partial pressure is important here because it applies to gases dissolved in liquids (e.g., blood) as well as in gas phase. As you’ll see in the rest of these notes, almost everything is about gas pressures! Partial pressures are very useful units in the respiratory system, because gases always diffuse down their partial pressure gradient.

How is the partial pressure of gas dissolved in a liquid defined? What is Henry’s Law and how does it apply to gas dissolved in liquid?
At equilibrium, gas dissolves into the liquid at the same rate that dissolved gas evaporates (leaves the liquid). If a liquid and a gas are at equilibrium, then the partial pressures of all gases in the liquid are equal to their partial pressures in the air (Pgas in liquid = Pgas in air). This is the definition of partial pressure.
Please remember that partial pressure is NOT equivalent to content. The partial pressure of a gas dissolved in a liquid is indeed an indication of the amount of gas there is. But the amount of gas dissolved in a liquid also depends on the solubility of the gas in that liquid. Different gases have very different solubilities! Henry’s Law states this mathematically: the amount of gas that dissolves (Cgas) depends on both partial pressure and solubility.
Attached figure:
The solubility of CO2 is higher than O2 so there is more in solution but partial pressures are still the same. if the partial pressure is higher in the air or the liquid, there will be a net movement of gas one way or the other until equilibrium is reached.

Using the attached graph, describe the physiologic importantance of PH2O in air.

The graphs depicts the temperature dependence of water vapor pressure when the air is saturated with water (i.e., the humidity is 100%). Water vapor pressure (PH2O) depends strongly on temperature. Air in the lungs is always at 37 degrees C – therefore PH20 is always 47 torr.
The capacity for air to contain water vapor decreases with decreasing temp. This is the reason water condenses on windshield from breath when it is cold
Using the attached table, explain why the PP of the respiratory gases change at different parts of the respiratory system.

The partial pressure of a given gas varies, depending on where you are in the respiratory system. However, the total pressure remains constant.
In round numbers (at sea level), air has PN2=600 torr, PO2 = 160 torr, PCO2 = 0 torr. When air is inspired, the body heats it to 37°C and humidifies it to 100% which is 47 torr water vapor. Air that is saturated with water vapor contains 47 torr water vapor at 37 degrees C (Fig. 32). Ambient air has less water vapor (depends on the humidity). The increase in water vapor pressure means other gases are displaced in the tracheal air. This displacement occurs in direct proportion to their partial pressures. Thus, PN2 decreases by 32 torr and PO2 decreases by 9 torr. Note that PCO2 in the air is negligibly small compared with other gases and compared with its much higher values in the lungs and airways.

We know that in the conductive zone of the respiratory system, gas moves by convection (bulk flow) and that in the respiratory zone, gas moves by diffusion. How large is the barrier to diffusion (without disease)? What makes up the barrier to diffusion?
Once gas is in the alveoli, it diffuses into alveolar capillaries. The key to this exchange lies in the structure of the diffusion barrier – it is less than 1 µm thick!!
The air-blood barrier consists of the alveolar epithelial cell (EP), capillary endothelial cell (E), interstitial space (IS), and intervening basement membranes (BM). Not visible in attached pic is the alveolar subphase – a thin layer of fluid on then alveolar surface. A layer of Pulmonary Surfactant one molecule thick lines the surface of the subphase fluid, at the air-liquid interface.
Note that diffusion is never rate limiting bc it is so fast over short distances.

How is the rate of diffusion calculated for gas exchange in the lungs? Discuss anatomical features of the lung that maximize the rate of diffusion.
See attached pic for equation for rate of diffusion.
Therefore, the rate of diffusion (Jgas) is proportional to the area (A) available for diffusion. This explains the benefits of the anatomical structure of the lungs, where the branching tree and the 300 million alveoli result in an area (A) roughly the same size as a tennis court. If your lungs were simply two balloons filled with air (like frog lungs), they would each have to be 12 feet in diameter to provide the same surface area for diffusion! The rate of diffusion is also proportional to the concentration gradient. When one talks about gas diffusion the relevant force is the partial pressure gradient. As you might remember, diffusion always occurs DOWN the partial pressure gradient (meaning from high to low partial pressure). In the lungs, a large partial pressure gradient is maintained by the residual gas at FRC – a buffer of sorts, keeping PA,O2 and PA,CO2 relatively constant. (This is because a normal VT exchanges only a fraction of the air in the lungs.)
In the tissues, diffusion also occurs according to Fick’s Law. If capillary blood has a low Pa,O2 (normally regulated at 100 mm Hg), then oxygen will not diffuse rapidly enough to the tissues (low PO2), and they will become hypoxic.

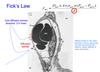
Explain oxygen equilibration btwn alveoli and the capillaries. Be sure to discuss partial pressures, solubility, and time for equilibration.
Diffusion across the alveolar-capillary interface happens quickly, and normally there is plenty of time for blood gases to equilibrate with alveolar gases. It takes about 0.75 seconds for blood to enter the dense meshwork of the alveolar capillary bed, flow past the alveolar surface, and exit the capillary on its way to the heart and eventually the tissues. Amazingly, ample diffusion takes place during this short period!
O2 diffuses through the air/blood barrier, and then diffuses into a RBC, chemically binding to hemoglobin. Once it enters the red cell and binds to hemoglobin, it no longer contributes directly to the partial pressure, thus maintaining the partial pressure gradient for further diffusion. Only dissolved O2 contributes to the partial pressure. More O2 molecules quickly enter and bind until the hemoglobin reaches a steady-state level of saturation. At less than half way through (0.25 seconds, see attached figure), Po2 in blood equilibrated with Po2 in alveolus. Could triple rate of pushing blood through lungs and blood would still get fully saturated with O2.
Note in the attached figure the equation for the rate of diffusion of O2. The low solubility of O2 in blood is made up for by the large driving pressure (difference in Po2 btwn air in alveolus and blood) such that the rate of diffusion is still very fast.

How is the time for equilibration of PO2 btwn alveoli and blood in capillaries impacted when diffusion is impaired (such as in pulmonary edema)?
How is the same parameter impacted when alveolar P)2 is abnormally low (such as at high altitudes)?
How does exercise affect O2 equilibration?
After 0.25 seconds in a normal person, the blood PO2 has already fully equilibrated with the alveolar PO2! Thus, in a normal individual, diffusion is rarely rate limiting. However, if diffusion is impaired (“abnormal” and “grossly abnormal” lines in Fig. 36, top panel-attached), as it is in edema (excess fluid in alveolar air space), full equilibration takes much longer than 0.25 seconds, if it occurs at all. When the alveolar PO2 is abnormally low (Fig. 36, bottom panel), such as occurs at high altitudes, it takes longer for the normal individual’s blood gases to equilibrate. This is because the alveolar-capillary gradient (PA,O2-Pv,O2) is decreased. The “abnormal” individual does not ever equilibrate when alveolar PO2 is abnormally low!
Heavy exercise increases cardiac output by up to a factor of five. However, the length of time when the RBC is flowing through the alveolar capillary bed is reduced – at most – to 0.25 seconds. This reflects recruitment and dilatation of pulmonary blood vessels at high BP,which we will discuss later. Even this is long enough for full equilibration. Note that there may be some diffusion limitation if a normal person exercises at high altitude. If an “abnormal” patient tries to exercise at high altitude, diffusion is significantly impaired.

Explain the equilibration of CO2 at the air-blood barrier in the lungs.
Carbon dioxide diffusion has a very similar overall time course to that of oxygen diffusion. This seems surprising, because the driving force – the partial pressure gradient is only ~5 torr for CO2, but is ~60 torr for O2. The diffusion coefficient for CO2 is not very different, 79% that of O2 (because CO2 is a larger molecule). However, Fick’s Law tells us that diffusion is also proportional to solubility. The solubility of CO2 is 24 times higher than the solubility of O2, which makes up for the smaller driving force (partial pressure gradient).
Bottom line: In normal individuals, blood is in the pulmonary capillary bed long enough and diffusion is fast enough for both O2 and CO2 to completely equilibrate across the air/blood interface.

How is the diffusing capacity of the lung measured? (equation). What is given to a pt to test this parameter?
See attached pic for equation.
DL is parameter that measures how well diffusion occurs between alveolar air and the blood. It incorporates everything from Fick’s Law, except driving force. DL is the rate a given lung takes up O2, (VO2) corrected for the driving force (PA,O2 - Pv,O2). DL is typically reduced in pulmonary disease.
In the denominator is the driving force. The driving force (PA,O2 - Pv,O2) does NOT determine DL! If you double the driving force, this will double VO2 but DL will remain constant (see pg 142 of notes).
CO transfer from air to blood is diffusion limited, so DL is usually measured with carbon monoxide (see attached), and is then called DL,CO. Because the goal is to understand how well diffusion occurs in the lung being tested, CO has ideal properties. Pv,CO is essentially zero, because any CO immediately binds tightly to Hb. This is bad for the subject, but good for the test, because it means nothing in the blood limits the rate of diffusion. Even if O2 could be used in this test, it would not give as much information, because as we saw, diffusion of O2 stops about 1/3 of the way through the alveolar capillary network, because it has fully equilibrated by then. CO keeps right on diffusing!
NOTE: Changing the driving force (PA,CO - Pv,CO) does NOT change DL,CO in a given subject!!! DL,CO reflects effectiveness of diffusion for a given driving force. Breathing faster, or with larger tidal volumes, or with a higher FiO2 has NO EFFECT on DL,CO!!!

Explain the changes in alveolar gases during breathing using the attached graph.
Why does PCO2 increase and PO2 decrease during the first part of inspiration?

Over the normal respiratory cycle, PA,CO2 and PA,O2 change as new air enters and gases diffuse. It should be noted that although alveolar PO2 and PCO2 do vary during each breath, this variation is small -only a couple of torr. The large volume of air that stays in the lungs (usually FRC, but down to RV during extreme breathing) acts as a “buffer” to prevent large changes in the alveolar gases. This buffering provides a safety factor for sub-optimal ventilation (or allows you to hold your breath for a while). In addition, by keeping the alveolar gases relatively constant, there is always a nearly constant and large partial pressure gradient to drive diffusion and gas exchange.
Why does PO2 decrease and PCO2increease during the first part of inspiration? Because of dead space. First part of inspiration is just air that was sitting in dead space. Once that is gone, begin to get new air into resp zone.
Use the attached graph to explain the effect of alveolar ventilation rate on the partial pressures of PO2 and PCO2.
What two factors determine alveolar gas content?

It might seem obvious that alveolar gas content depends on ventilation. But it also depends on metabolism (O2 consumption and CO2 production) of the organism. For any given metabolic rate, alveolar ventilation VA (not total minute ventilation, VE) will establish the alveolar gas levels.
The attached figure assumes a constant metabolic demand. Clearly, increasing VA increases PA,O2 and decreases PA,CO2. An important point is that doubling VA decreases PA,CO2 by ½ but does NOT double PA,O2. This is understandable if you remember that increasing VA results in both of the alveolar gases approaching their values in inspired air (PI,O2 = 150 torr, PI,CO2 = 0 torr). If PA,O2 is already 100 torr, you cannot double it– the highest PA,O2 you can get at sea level is 149 torr. In contrast, PA,CO2 can decrease essentially to 0. Each time you double ventilation, you increase PA,CO2 by 50%.

What is the alveolar gas equation? What is it used to determine?



