Unit 2 Exam Flashcards
(54 cards)
Daltons Atomic Theory
- All atoms of a given element are identical (except Isotopes)
- Atoms of different elements have different masses
- compounds are made of different elements combined in specific ratios
- in a chemcal reaction atoms are neither created or destroyed
Proton
Postively Charged particle
Neutron
nuetral particle
electron
negatively charged
Necleus Characteristics
- contains protons and neutrons
- positive charge
- determines mass of atom
- extremely small, but dense
Mass #
of protons plus # of neutrons

Atomic Number
Number of Protons
How to determine the # of neutrons
Mass # - atomic #
Determine the # of electrons
of protons for a neutral atom
Isotope
Something has the same # of protons but different # of neutrons

Ion
charged particle formed by gain or loss of electron
(+) ion loss of electron
(-) ion gain of electron
J.J. Thomson (1897)
Determined the charge to mass ratio of an electron
used the cathode ray tube (An electric field is set up between 2 plates, + and - charged and a magnetic field is applied perpendicular to the electric field and the electrons are fed between the the two plates)
Found that the charged particles (electrons) were the same regardless of the metal he used for the cathode.
Concluded that electrons are part of the makeup of all atoms

Milliken Oil Drop Exp (1909)
Determined the charge of electron then using thomsons value calculated mass of an electron
Observed tiny electrically charged oil droplets. From the strength of the electric field required to overcome the pull of gravity on the droplets, he determined the values of the charges on the particle.
Experiment Setup: oil is sprayed as a fine mist into a chamber containing a charged gas, and the location of an oil drop is monitored by using a microscope. Charged particles (ions) are generated in the gas by exposing it to x-rays. The fall of the charged droplet is balanced by the electric field.

Ernest Rutherford Exp (1908)
Determined nucleus (+) charge and very dense and atom is mostly empty space.
Experiment required shooting alpha particles toward a piece of platinum foil only a few atoms thick. Found almost all alpha particles passed through and were deflected only slightly (about 1 in 20,000 deflected more than 90 degrees). some bounced back straight in direction they had come.
When a positively charged alpa particle scored a direct hit on one of the minute but heavy platinum nuclei, the alpha particle was strongly repelled by the positive charge of the nucleus and deflected through a large angle.

mass spectrometer
can measure the mass of isotopes and % abundances
Deflection depends on:
- Magnitude of acclerating voltage
- higher voltage —-> beam moves more rapidly
- less deflection
- higher voltage —-> beam moves more rapidly
- magnitude field strength-stronger field deflects beam more
- masses of particle-heavier particles deflected less
- charges on particles
- higher charges are deflected more

Steps for calculating average atomic mass
- change % to decimal
- multiply by mass
- Add
Wavelength
distance it takes for wave to repeat itself
C=λv
C=speed of light
v=frequency (given in Hz which is = to cycles/sec or s-1)
Energy Equation
E=hv
E=energy
h=planks constant
v=frequency
Neils Bohr (1930’s?)
Said electrons have a quantum value—can only have certain values
farther from the necleus—-higher the energy
there are places you expect to find atoms then gaps where you don’t
Balmer Series
visible spectrum
n1 = 2
Lyman Series
ultra-violet
n1 = 1
Bohr Frequency condition
V=Z2R( 1/n12 - 1/n22)
R = Ryberg constant
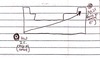
Photoelectric Effect
shows the particle (photon) nature of electromagnetic radiation
When light of sufficient energy strikes the metal, an electron is knocked off, it travels to the positive electrode and current flows to the circuit. ( see image)

Classical Theory
If low levels of light are emitted long enough then it should
EK = 1/2 mv2 = hν−ϕ
ϕ = energy needeed to remove electron work function
hv= energy supplied by photon