Topic 17+18: Further Organic Flashcards
What is optical isomerism?
Stereoisomerism has 2 types. Ya sabes geometric, now learn optical.
Optical isomers: non-superimposable mirror images of each other w asymmetry. This is chirality.
Enantiomers: chiral molecules w C as the chiral centre.
Chirality arises if a molecule contains a tetrahedral C atom w 4 DIFF groups attached. If 2 groups are the same, there’s no chirality bc one can be superimposed on the other.

What is the difference between an unpolarised wave and a plane polarised wave?
Unpolarised wave: oscillations may occur in any plane or direction.
Plane polarised wave: oscillations only occur in 1 plane/direction.
Some materials can absorb all of the oscillations except those in a single plane, and so convert unpolarised light into plane polarised light.
Describe and explain polarimetry and how a polarimeter works
Polarimetry: using a polarimeter to measure the amount of optical activity. A monochromatic light passes thru a polarising filter called the polariser. This converts unpolarised light into vertically plane polarised light.
Plane polarised light passes thru a sample tube w some of the substance in solution. If the substance is optically active (bc it is an enantiomer), then the plane of polarisation rotates. Clockwise rotation means the substance is dextrorotary. Anticlockwise rotation= laevorotatory.
The second polarising filter (the analyser) is rotated to a position where max light intensity can be seen. The angle of rotation is measured. It is + if the rotation is clockwise and negative if the rotation is anticlockwise.
Describe physical and chemical properties of two enantiomers.
2 enantiomers= identical phys properties except, they rotate plane polarised light by equal angles, but opp directions. They’re thus optically active. So if rotation for 1 enantiomer is +60°, rotation for the other is -60°.
2 enantiomers have identical chem properties w 1 exception: the way in which they react with enantiomers of other substances. This property may be diff for each enantiomer.
When hay equal quantities of 2 enantiomers the mixture is racemic. No hay optical activity bc the anticlock and clockwise rotations on plane polarised light cancel out.
Draw the SN2 mechanism for nucleophilic substitution of 3-fluro-3-bromoethane and explain the product formed.
This is an enantiomer. Only 1 product isomer is formed. If you had 1 enantiomer to begin w, you end up w the opp one in the product. Eg if reactant=dextrorotary, product= laevorotary.
By measuring the optical activity of the original haloalkanes and the alcohol formed, we can show if the reaction has occurred by the SN2 mechanism.

Draw the SN1 mechanism for 3-fluro-3-bromoethane
The og haloalkane has a tetrahedral shape, but the product of the first step is a planar carbocation.
This means that in the 2nd step hay an equal chance that the attacking HO:- can approach from the left OR right. Therefore hay 2 enantiomer products. These are present in equal numbers so a racemic mixture is formed. The product will have no optical activity.

Describe polar bonding in carbonyls
The electron density in the pi bond alkenes are evenly distributed across both carbon atoms.
In carbonyls however, the C=O bond is polar bc of differing electronegativities of carbon and oxygen. The electron density is greater near the oxygen rather than the carbon atom.
Describe bpt of carbonyls
Aldehydes and ketones have higher intermolecular forces than alkanes.
They contain the polar C=O group so they have permanent dipole-dipole attractions. No H bonding as all their H atoms are joined to C atoms.
As with alkanes and alcohols, boiling temps increase w increasing chain length (more Ldn forces). At room temp methanal is a gas. The other carbonyl compounds are liquids.
Describe solubility of carbonyls
Lower aldehydes and ketones are soluble in water because they can form H bonds with water molecules.
Solubility of aldehydes and ketones decreases with increasing chain length as the hydrocarbon part of the molecule increases and is not polar.
How can aldehydes and ketones can be reduced to alcohols?
Both aldehydes and ketones can be reduced to alcohols by LiAlH4.
Conditions: both the carbonyl compound and reducing agent are dissolved in dry ether. The reducing agent is represented by H.
CH3CH2CHO + 2[H] –> CH3CH2CH2OH
CH3COCH2CH3 + 2[H] –> CH3CH(OH)CH2CH3
Describe how to distinguish between aldehydes and ketones using Acidified potassium dichromate.
Acidified potassium dichromate heated under reflux is used to test for alcohols (1y and 2y) & aldehydes
The colour goes from orange Cr2O72- to green Cr3+ Reduced from Cr(+6) to Cr(+3)
Describe how to distinguish between aldehydes and ketones using Fehlings/Benedict’s solution.
Reagents: copper sulphate solution & NaOH solution. Conditions: warm gently
Aldehydes form a brick red ppt, ketones don’t form a brick red ppt. Aldehydes reduce the Cu(II) ion to Cu(I) oxide.
Because the solution is alkaline, the aldehyde itself is oxidised to a salt of the corresponding carboxylic acid. RCHO + 2Cu 2+ + 5OH- -> RCOO- + Cu2O + 3H2O
Describe how to distinguish between aldehydes and ketones using Tollen’s reagent
This is an oxidation reaction, Tollen’s is the oxidising agent
Reagents: silver nitrate solution, few drops of NaOH (aq) and a little ammonia solution. Warm gently.
Result: Aldehydes: silver mirror Ketones: no silver mirror
Aldehydes reduce the diamine silver(I) ion to metallic silver. Because the solution is alkaline, the aldehyde is oxidised to a salt of the carboxylic acid.
Describe how to distinguish between aldehydes and ketones using the iodoform reaction
Reagents: solution of iodine in potassium iodide & NaOH solution. Conditions: cold/room temp.
Positive result = yellow ppt and antiseptic smell
This shows the presence of a C=O next to a methyl group (we call it a methyl carbonyl) OR an –OH next to a terminal CH3 group
Describe how to detect a carbonyl using 2,4-DNP (Brady’s reagent)
Reagents: 2,4 DNPH Conditions: room temp. Result: yellow/orange ppt.
The reaction is a condensation reaction bc 2 molecules join together with the loss of a small molecule, in this case water. (nucleophilic addition-elimination reaction).
To determine WHICH carbonyl was present: the ppt is filtered off, recrystallised and the mpt found. This can be compared to known mpts of 2,4-DNPH derivative to identify the original carbonyl.
Describe reactivity of aldehydes vs ketones
Aldehydes are generally more reactive than ketones bc the presence of the two alkyl groups hinders nucleophilic attack and reduces the partial + charge on the carbon atom of the carbonyl group.
What is recrystallisation?
Recrystallisation purifies a solid substance. When a compound is isolated from a reaction mixture it contains impurities which are removed by dissolving the substance in a suitable hot solvent and leaving to cool.
As the solution cools the solubility of the compound decreases and purer crystals are formed. Recrystallisation may be repeated several times.
Impurities in a substance will affect its mpt and make it difficult to identify the compound isolated from a reaction.
Why is HCN so dangerous?
Highly toxic because it inhibits a mitochondrial enzyme for restoration.
It is hard to handle safely because it’s so volatile and flammable.
A safer alternative is KCN which is solid at room temp and it’s easier to handle. An acidified solution contains both H+ and CN- ions
How do carbonyls react w hydrogen cyanide?
The reaction is carried out in aq alkaline solution of sodium cyanide. The equations/mechanisms are the same for aldehydes and ketones. A hydroxynitrile forms.
A H atom attaches to the O of the carbonyl group and a CN group attaches to the carbon atom of the carbonyl group. Addition to propanal forms 2-hydroxybutanenitrile CH3CH2CHO + HCN –> CH3CH2CH(OH)CN
Addition to butanone –> 2-hydroxy-2-methylbutanenitrile CH3COCH2CH3 + HCN –> CH3C(CN)(OH)CH2CH3
Draw the mechanism for the reaction : nucleophilic addition of KCN to ethanal

Describe the melting and boiling pts of carboxylic acids
High bpts due to H bonding (higher than alkanes).
Boiling temperatures increase with increasing molar mass because of more London forces.
Describe solubility of the carboxylic acids
Small carboxylic acids are soluble in water. H bonds form between water and carboxylic acid molecules.
Solubility of the bigger acids decreases rapidly w size. Longer hydrocarbon part of the molecule increases and is not polar.
What kind of acids are carboxylic acids?
Carboxylic acids are weakly acidic bc of the slighlty + H in the COOH.
The - charge is delocalised across the carboxylate group resulting in a more stable ion. Delocalisation is represented by adding a dotted line to the CO bonds which are both equivalent.
So carboxylic acids PARTIALLY dissociate in aq solution

How are carboxylic acids made weaker and stronger?
Electron donating groups push e towards the carboxylic acid carbon, which stabilises the OH bond and makes it harder to break. EDG make acids weaker, hay less dissociation so Ka is smaller
Electron withdrawing groups eg halogens or electroneg groups pull e away from the carboxylic acid carbon, weakening the OH bond, making it easier to break. EWG make acids stronger, hay mas dissociation, Ka is bigger
CH3COOH + H20 → CH3C00- + H3O+
In short: Methyl groups donate/push e-s away and stabilise neighbouring carbons. Halogens/electroneg groups withdraw electrons away.
State the 3 ways you can make carboxylic acids
1) strong oxidation of a primary alcohol w acidified k dichromate under reflux
2) further oxidation of an aldehyde
3) hydrolysis of a nitrile. Reagents: dilute HCl/H2SO4. Conditions: boil/reflux
CH3CN + 2H2O + H+ → CH3COOH + NH4+
what are amines?
Amines: organic compounds based on ammonia, NH3 , in which one or more of the hydrogen atoms are replaced by alkyl or aryl groups.
Amines have a fishy or rotting smell

what is the shape of amonia?
Ammonia (NH3) is pyramidal in shape, as it has a lone pair in place of one bonding pair.
These exert a stronger repulsive force, so push the bonding pairs further away.
This reduces the bond angle by about 2.5°
How do we name primary amines?
C2H5NH2 ethylamine
C4H9NH2 butylamine
C6H5NH2 phenylamine

How do you name secondary amines?

How do you name tertiary amines?
Tertiary: trialkyl prefix if chains same, e.g. trimethylamine
OR: N,N-alkylalkylalkylamine
e.g. N,N-ethylmethylpropylamine

Name the following:


What happens when other NH2 functional groups are present in the amine?
If other functional groups are present in the molecule, the presence of amine groups is denoted using the amino– prefix.

Describe bpts of amines
Primary and secondary amines have a polar N–H bond so they can form H bonds w other amine molecules.
Primary have 2 H-bonds, so would have the higher mpt than secondary
Tertiary amine molecules have no H atoms directly attached to the N so no H bonds. Therefore tertiary has a lower bpt
Explain amine solubility in water.
Water can form H bonds with all amines, including tertiary amines (most with primary then secondary then tertiary).
Thus smaller amines are readily soluble in water but solubility decreases as the number of C atoms increases bc larger R groups interfere with the hydrogen bonds.
How do you form aliphatic primary amines using ammonia?
Nucleophilic sub reaction of a haloalkane and conc. aq ammonia forms primary amine and hydrogen halide:
CH3CH2Cl + NH3 → CH3CH2NH2 + HCl
BUT, the primary amine formed can then attack another halogenoalkane molecule to form secondary amine (further reactions continue to produce tertiary amines and so on):
CH3CH2NH2+ CH3CH2Cl → (CH3CH2)2NH + HCl
A mixture of products is produced so method is rarely used
How do ypu form aliphatic primary amines using EXCESS ammonia?
React haloalkanes w EXCESS ammonia.
The HCl made in the first step reacts with excess ammonia to make ammonium chloride.
This reduces the ammount of unwanted side products forming when reacting with ammonia which is not in excess.
CH3CH2Cl + 2NH3 → CH3CH2NH2 + NH4Cl
Describe a second way of making primary amines
Reduction of nitriles to make amines. This can be done in 2 ways:
With a reducing agent, e.g. LiAlH4
Or with heat, hydrogen and a nickel catalyst (like hydrogenation of the triple bond)
This is the preferred method as only one amine formed
Describe the base strength of amines
Amines= weak bases bc the lone pair on N can accept a proton by forming a dative cv bond to the H+.
Base strength depends on how well N lone pair can accept H+. The higher the e density of the N lone pair, the stronger the base because there is greater ability to accept H+
ALKYL groups push electrons towards the N better than H because of carbocation stability.
In aromatic amines, the N lone pair is partially delocalised into the benzene ring, lowering the base strength even further.
so tertiary is strongest, then s, then p, then NH3 then aromatic amines .
Describe general Amine/acid neutralisation reactions
The amine + acid forms ammonium salt and water. Amines accept H+ from acids to form quarternary ammonium salts: CH3CH2NH2 + H+ Cl- → CH3CH2NH3 + Cl-
If the reaction is carried out in solution, the amine accepts a H+ from H3O+ to form an ionic salt and water (neutralization):
CH3CH2NH2(aq) + H3O+(aq) + Cl-(aq)→ CH3CH2NH3+Cl-(aq) + H2O(l)
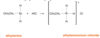
draw the reaction for methylamine and nirtic acid

Draw the reaction for diethylamine and sulfuric acid

Describe amine reactions with water
Amines are soluble in water, but they also react with water (like NH3): amine + water → ammonium ion + OH-

What are Quaternary ammonium salts used for?
Quaternary ammonium salts used as cationic surfactants in fabric conditioner and shampoo.
They soften fabric/hair by reducing surface tension.
used as antiseptics in products such as wet wipes.
Draw the mechanism for amine + haloalkane, and describe any products/ conditions
This is a nucleophilic substitution reaction. Heat in ethanol in sealed tube
R’NH2 + R’’X → R’NHR’’ + HX
The product also has lone pair on N, so again, this reaction can continue so secondary product can react further to tertriary, to ammonium salt.

Describe the reaction of amines with Cu2+ ions
Amines have a N with a lone pair so they form dative bonds with transition metals in complex ions
On adding an amine to Cu2+, a pale blue ppt is produced in an acid base reaction:
[Cu(H2O)6]2+ + 2RNH2 → Cu(H2O)4(OH)2 + 2RNH3+
Adding excess amine forms a dark blue solution:
Cu(H2O)4(OH)2 + 4RNH2 → [Cu(RNH2)4(H2O)2]2+ + 2OH- + 2H2O
The amine replaces the 2OH- ions and 2 water ligands in the complex ion in a ligand exchange reaction.
Why is phenyamine more reactive than Benzene?
More reactive than benzene for the same reason as why phenol is more reactive than benzene (lone pair delocalised into ring, ‘activates’ ring so it is more attractive to electrophiles)
It is more susceptible to electrophilic attack at positions 2, 4 and 6.
describe AMIDES
These are derivatives of carboxylic acids. The functional group is the amide group: -CONH2
Amides are generally white crystalline solids, and the smaller ones are soluble in water due to H bonding
How are amiDES formed?
Amides can be formed by reacting: A carboxylic acid with an amine OR An acyl chloride with an amine
Acyl chlorides are more reactive so these are preferred in many reactions.

Draw the mechanism for forming an AMIDE

How do you prepare N substitued AMIDES

How do u form POLYAMIDES
If you have a molecule with a carboxylic acid/acyl chloride at one end, and an amine at the other, these can form an amide.
If your molecules have functional groups at BOTH ends, they can carry on reacting, forming big long chains of molecules joined together through amide links:
diacid monomer + diamine monomer → poly (amide)
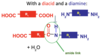
Give properties of polyamides
High tensile strength, resistant to most chemicals (except acid) and high temps. This makes them great for clothing!
Kevlar is used to make bullet-proof vests
How can amides be broken up?
Amides can be broken up into their component acid and amine by hydrolysis: CH3CONH2 + H2O→ CH3COOH + NH3
This can be done w acid, forming ammonium salt as a product: CH3CONH2 + H2O + HCl → CH3COOH + NH4Cl
OR alkali: CH3CONH2 + NaOH(aq) → CH3COONa + NH3
How can we test for amides?
Warming an amide with dilute NaOH solution and testing for ammonia using moist red litmus paper.
What is special about amino acids?
The carboxyl group means it can behave as an acid and the NH3 amine group means it can behave as a base.
These groups can also mean amino acids react as amines and carb acids.
aa have chirality.
Describe how amino acids react
In water: R-NH2 + H20 –> RH3+ + OH-
R-COOH + H20 –> RCOO- + H+
In acid: R-NH2 + H+ —> RNH3+
In alkali: R-COOH + OH- –> RCOO- + H20
From the acid alkali reaction the products RNH3+ and RCOO-would react together to form salts. Therefore aa are amphoteric.
What are zwitterions?
Zwitterions have both a + and - charge which cancel out. The H from the COOH is donated to the N on NH2.
Will only form at the isoelectric point.
Below the isoelectric point (acidic pH) zwitterions act as a base and accept protons. Above the isoelectric point (alkaline pH) they act as an acid and donate protons

How are proteins hydrolysed?
Prolonged heating was a concentrated HCl. Bc of the strongly acidic conditions all amino acids formed will have the NH2 groups protonated:

Describe combustion and addition reactions of aromatic compounds
Aromatics burn with a smoky flame because of the high ratio of C:H
In aromatics, the delocalised pi e- ring means benzene is v stable. Undergoing addition reactions would break the pi system so addition is unfavourable.
Instead they undergo substitution bc the delocalised pi ring is maintained. Therefore benzene doesn’t decolourise Br water react w strong acids/halogens
Describe electrophilic substitution in aromatic compounds
In aromatics, the 6 e- in the pi system are delocalised over the 6 Cs in the double bond. Therefore e- density is less than an alkene. It cannot induce the dipole in a halogen. You need a catalyst to create this dipole.
Benzene will react with an electrophile if the elctrophile is generated first. free electrons means benzene is electron rich and therefore attracts electrophiles. The reaction that follows is electrophilic substitution.
Describe the conditions required for nitration of benzene.
Conc HNO3 and conc H2SO4 (aka the nitrating mixture), 50°C. More N02 groups can be subbed if mix is hotter. To ensure mono substitution place the mix in an ice bath.
Nitrobenzene is used in dyes, pesticides pharmaceuticals. As benzene can’t induce a dipole the NO2+ electrophile is made by the nitrating mixture.
Nitration of METHYLlbenzene: this reaction is faster and can form 2,4,6trinitromethylbenzene (TNT)

How is nitrobenzene used to make an aromatic amine?
Nitrobenzene + tin + conc HCl heated under reflux act as reducing agents.
Followed by NaOH to neutralise excess H+
Phenylamine is formed which is separated from the mix

Describe halogenation of benzene
Bc benzene can’t induce a dipole (too stable), halogens dont react w it unless a halogen carrier catalyst, eg AlCl3 or FeBr3 is used. This polaries the halogen and thus forms an electrophile.

Describe alkylation of benzene
Alkylation is a type of Friedel-Crafts reaction. Used to sub any alkyl R grup onto benzene:

Describe acylation of benzene using an acyl chloride
Acylation substitutes a COR group onto the benzene ring. It is an electrophilic substitution reaction done under reflux.

Draw the mechanism for acylation of benzene w an acid anhydride
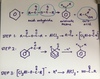
Describe phenol (aka aromatic alcohols)
OH group is attached directly to benzene. A pink solid at room temp, used to make antiseptics and disinfectant.
The lone pair of e- on the oxygen are also in a P orbital so they’re drawn into and become part of the delocalised benzene ring. This increases e- density in the ring and makes the ring more susceptible to electrophilic attack. It also makes the OH’s hydrogen a lot more acidic.
Mpt=40°C bpt=182°C due to H bonding (more than benzene) moderately soluble in water bc of H bonds.
When dissolved in water final becomes a weak acid by losing H+ ions from the OH group.
Does phenol react with bromine water?
Unlike benzene, phenol reacts w a solution of Br water at room temp sin catalyst
2,4,6-tribromophenol (white ppt) is formed. The electrophile is formed from the reaction of Br w water.

Describe the reaction of phenol with dilute and conc nitric acid
Phenol reacts w dilute HNO3 whereas benzene needs a nitrating mixture of conc nitric acid and conc H2S04. This is bc one of the lone pairs on the oxygen atom in the OH group overlaps with the delocalised ring of e-, making phenol mucho mas reactive.

Describe reactions of phenol with sodium and sodium hydroxide
Bc it can act as a weak acid, phenol reacts w bases in neutralisation reactions.
Phenol + NaOH forms sodium phenoxide which is soluble in water. The reaction can be reversed by adding a strong acid.
Phenol + Na forms sodium phenoxide and hydrogen gas

Describe limitations of the Kekule structure for benzene
The Kekule structure shows 3 C=Cs which isn’t accurate bc the bonds would have to be diff lengths. 6 e- of the carbon are delocalised in pi bonds in benzene but not structure K.
What happens when an alcohol reacts with a carboxylic acid?
Alcohol + carboxylic acid –> ester + water.
Conditions: heat under reflux w conc H2SO4 catalyst. The reaction is reversible and occurs very slowly. In industry esters are used as solvents and making polyesters.

Describe acyl chlorides
General formula RCOCl. The C atom in COCl is joined by 2 electroneg atoms so it’s Delta +. Therefore it’s readily attacked by nucleophiles.
They have low bpts bc no H bonding and fewer e-s, so fewer ldn forces. Don’t rlly dissolve in water, just vigourously react w it. However it’s polar so has dipole dipole attractions and Ldn forces. bpt = 51 degrees C
Describe the reaction of acyl chloride + water
Describe the reaction of acyl chloride + alcohol using ethanoyl chloride + ethanol as an example
Carboxylic acid and HCL gas formed. Eg ethanoyl chloride + H20 –> ethanoic acid + HCl gas
Ethanoyl chloride + ethanol –> ester + HCl gas. Cold conditions. Mechanism= nucleophilic addition elimination.

Describe the reaction of acyl chloride with concentrated ammonia
Ethanoyl chloride + NH3 –> CH3C=ONH2 (ethanamide) + HCl
The reactant is a base and the product is an acidic gas which react together to form ammonium chloride. A single equation combines both of these reactions:
CH3COCl + 2NH3 –> CH3CONH2 + NH4Cl
Describe the reaction of acyl chloride w an amine
N-substituted amide. Secondary amines react the same, but the product sera two substituted alkyl groups
This reaction does not work w tertiary amines. W 3 Alkyl groups no hay hydrogen atoms to react with chlorine to form HCl.

Describe the reaction of acyl chloride with phenylamine

What is an acid anhydride?
It’s forms from a condensation reaction between two carboxylic acids
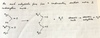
How can acid anhydride be used to make esters?
Acid anhydride + alcohol –> Ester + carboxylic acid

How can you make esters from phenol?
OH of phenol is less reactive than OH of ethanol since its lone pair is delocalised onto the ring, stabilising it.Therefore only a more vigourous acylating agent needed to make the ester:
Phenol + acyl chloride —> ester + HCl
Phenol + acid anhydride —> ester + Carb. acid
Esters are colourless liquids w fairly low mpts, insoluble in water. No H bonding. Used in perfume, flavourings, solvents.
Describe hydrolysis of esters/ polyesters
Ester + water OR dilute acid –> carboxylic acid + alcohol
Hydrolysis w pure H20 is so slow that it’s never used. For acid, the ester is heated under reflux w dilute HCl or H2SO4. Reactions are reversible, hydrolysis is incomplete.
Ester + dilute alkalai –> Na -oate + alcohol. Heat under reflux. Irreversible, hydrolysis is complete. You get a sodium salt rather than a carb acid so the products are easier to separate. Only add acid to convert the salt to a carboxylic acid.

Describe polyesters, using terephthalic acid as an example
Flammable, makes packaging, clothing.
To produce a polymer you need 2 monomers w 2 reactive groups at each end, eg a diol or dicarb. acid.
The larger molecule formed still has reactive groups at both ends so it can continue reacting to form a long chain polymer. See image for the structure of the polymer.
It’s possible to use a dioyl chloride instead of a dicarboxylic acid but the small molecule formed is HCl, not H20.
What are Grignard reagents and how are they made?
Haloalkane + magnesium → Grignard reagent (R-Mg-X)
This reaction must be done in dry ether. Grignard reagents extend the C chain. After the reaction is complete, dilute acid is added to obtain the desired product (acid hydrolysis)
RMgX + CO2 → RCOOH
RMgX + CH2O → RCH2OH (primary alcohol)
RMgX + R’CHO → RCH(OH)R’ (secondary alcohol)
RMgX + ketone → (tertiary alcohol)
How do you form 3-methylbutanoic acid using a Grignard reagent?
The COOH part comes from C02. The starting compound therefore supplies the (CH3)2CHCH2 by using the haloalkane (CH3)2CHCH2Br.

How do you form propan-1-ol w a Grignard reagent?

How do you form pentan-2-ol and 2-methylpropan-2-ol w a Grignard reagent?



