Test 6 ETC and OP Flashcards
(16 cards)
Summary of Electron Transport Chain
Electron Transport: Electrons carried by reduced coenzymes are passed through a chain of proteins and coenzymes to drive the generation of a proton gradient across the inner mitochondrial membrane
The mitochondrial electron transport chain (ETC) carries out the following two reactions:
- NADH +O2 -> NAD+H2O DG= -56kcal/mol
- FADH2 +O2 -> FAD+H2O DG= -42kcal/mol
Delta G indicates the large amount of energy released by both reactions. (No need to memorize values)
(D stands for delta)
Important 3 Points
Four protein complexes in the inner mitochondrial membrane
A lipid soluble coenzyme (UQ, CoQ) and a water soluble protein (cyt c) shuttle between protein complexes
Electrons generally fall in energy through the chain - from complexes I and II to complex IV

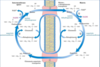
Malate Shuttle (Malate-Aspertate Shuttle)
*Know the mechanism and function*
Summary: If NADH is produced in the cytoplasm,
- the malate shuttle or the alpha-glycerol phosphate shuttle can transfer the electrons into the mitochondria for delivery to the ETC.
- Once NADH has been oxidized, the NAD can again be used by enzymes that require it.
- Malate-aspartate shuttle uses malate to carry electrons across the membrane
Mechanism:
1) NADH+H+ in the cytosol (intermembrane space) passes two reducing equivalents to oxalacetate, producing malate.
2) Malate is transported across the inner membrane by malate/a-ketoglutarate transporter.
3) In the matrix, malate passes two reducing equivalents to NAD+; the resulting matrix NADH+H+ is oxidized by the mitochondrial respiratory chain. The oxalacetate formed from malate cannot pass directly into the cytosol.
4) It is first transaminated to form asparate, –> which can leave via the glutamate/asparate transporter.
5) Oxalacetate is regenerated in the cytosol completing the cycle.
*32 ATP per glucose if malate-asp shuttle used*
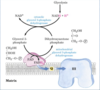
Glycero-Phosphate Shuttle
FADH2 is produced by succinate dehydrogenase in the TCA and by the a-glycerol phosphate shuttle.
- Both enzymes are located in the inner membran ę and can reoxidize FADH2 directly by transferring electrons into the ETC.
- Once FADH2 has been oxidized, the FAD can be made available once again for use by the enzyme.
- Glycerophosphate shuttle stores electrons in glycerol-3-P, which transfers electrons to FAD
This is an alternative means of moving reducing equivalents from the cytosol to the mitochondrial matrix.
1) Dihydroxyacetone phosphate in the cytosol accepts two reducing equivalents from cytosolic NADH+H+ in a reaction catalyzed by cytosolic G-3-P dehydrogenase.
2) A membrane-bound isozyme of G-3-P dehydrogenase, located on the outer face of the inner membrane, transfers two reducing equivalents from glicerol-3-phosphate in the intermembrane space to ubiquinone.
- This shuttle does not involve membrane transport systems.
- 30 ATP per glucose if glycerol-3-P shuttle used
Oxidative Phosphorylation
- ATP synthesis by oxidative phosphorylation uses the energy of the proton gradient and is carried out by the F0F1 ATP synthase complex, which spans the inner membrane
- As protons flow into the mitochondria through the F 0 component, their energy is used by the F 1 component (ATP synthase) to phosphorylate ADP using Pi
- On average, when:
> NADH is oxidized in the ETC, sufficient energy is contributed to the proton gradient for the phosphorylation of 3 (2.5) ATP by F0F1 ATP synthase
> FADH2 oxidation provides enough energy for approximately 2 (1.5) ATP.
> These figures are referred to as the P/O ratios.

Reduction Potentials
*Crucial Equation*
For more infromation refer to Slide #10
- High Eo’ indicates a strong tendency to be reduced*
- Crucial equation: DGo’ = -nF DEo’
- DEo’ = Eo’ (acceptor) - Eo’ (donor)
- Electrons are donated by the half reaction with the more negative reduction potential and are accepted by the reaction with the more positive reduction potential: DEo’ positive, DGo’ negative
- If a given reaction is written so the reverse is true, then the DEo’ will be a negative number and DGo’ will be positive
Complex I
NADH-CoQ Reductase (other name)
- Electron transfer from NADH to CoQ
- More than 30 protein subunits - mass of 850 kD
- Path:
- NADH > FMN > Fe-S > UQ> FeS > UQ
- Four H+ transported out per 2 e-
Complex II
Succinate-CoQ Reductase (other name)
- aka succinate dehydrogenase (from TCA cycle!) aka flavoprotein 2 (FP2) - FAD covalently bound four subunits, including 2 Fe-S proteins
- Three types of Fe-S cluster:
- 4Fe-4S, 3Fe-4S, 2Fe-2S
- Path: succinate > FADH2 > 2Fe2+ > UQH2
(Important Pathway memorize) ^^^
Net reaction:
-> succinate + UQ -> fumarate + UQH2

Complex III

CoQ-Cytochrome c Reductase
- CoQ passes electrons to cyt c (and pumps H+) in a unique redox cycle known as the Q cycle
- The principal transmembrane protein in complex III is the b cytochrome - with hemes bL and bH
- Cytochromes, like Fe in Fe-S clusters, are one- electron transfer agents
- Study Figure 17.12 - the Q cycle (17.12a is on front of card, 17.12b is on this side)
- UQH2 is a lipid-soluble electron carrier
- cyt c is a water-soluble electron carrier

Complex IV
Cytochrome c Oxidase
- Electrons from cyt c are used in a four- electron reduction of O2 to produce 2H2O
- Oxygen is thus the terminal acceptor of electrons in the electron transport pathway - the end!
- Cytochrome c oxidase utilizes 2 hemes (a and a3) and 2 copper sites
- Structure is now known - mostly!
- Complex IV also transports H+
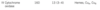
Table 19.2

Table 19.4

Table 21.4

Reactive Oxygen Species (ROS)
- When molecular oxygen (O2) is partially reduced, unstable products called reactive oxygen species (ROS) are formed.
- These react rapidly with
> lipids to cause peroxidation,
>with proteins, and with other substrates, resulting in denaturation and precipitation in tissues.
- Reactive oxygen species include:
- Superoxide (O•2-)
- Hydrogen peroxide (H2O2)
- Hydroxyl radical (OH•)
Reactive Oxygen Species (ROS) Slide 64
- The polymorphonuclear neutrophil produces these substances to kill bacteria in the protective space of the phagolysosome during the oxidative burst accompanying phagocytosis.
- Production of these same ROS can occur at a slower rate wherever there is oxygen in high concentration.
- Small quantities of ROS are inevitable by-products of the electron transport chain in mitochondria.
- These small quantities are normally destroyed by protective enzymes such as catalase.
- The rate of ROS production can increase dramatically under certain conditions, such as reperfusion injury in a tissue that has been temporarily deprived of oxygen.
- ATP levels will be low and NADH levels high in a tissue deprived of oxygen (as in an MI).
- When oxygen is suddenly introduced, there is a burst of activity in the ETC, generating incompletely reduced ROS.
Reactive Oxygen Species (ROS) Slide 65
- Defenses against ROS accumulation are particularly important in highly aerobic tissues and include:
superoxide dismutase and
cataIase.
- In the special case of erythrocytes, large amounts of superoxide are generated by the spontaneous dissociation of the oxygen from hemoglobin (occurrence is 0.5-3% of the totaI hemoglobin per day).
- The products are methemoglobin and superoxide.
- The processes that adequately detoxify the superoxide require a variety of enzymes and compounds, including:
superoxide dismutase,
catalase,
glutathione peroxidase,
vitamin E in membranes,
vitamin C in the cytoplasm.
- Low levels of any of these detoxifying substances result in hemolysis.
- For example, inadequate production of NADPH in glucose 6-phosphate dehydrogenase deficiency results in accumulation of the destructive hydrogen peroxide
Coordinate Regulation of the Citric Acid Cycle and Oxidative Phosphorylation
- The rates of oxidative phosphorylation and the citric acid cycle are closely coordinated, and are dependent mainly on the availability of O2 and ADP.
- If O2 is limited, the rate of oxidative phosphorylation decreases, and the concentrations of NADH and FADH2 increase.
- The accumulation of NADH, in turn, inhibits the citric acid cycle.
- The coordinated regulation of these pathways is known as “respiratory control.”
- In the presence of adequate O2, the rate of oxidative phosphorylation is dependent on the availability of ADP.
- The concentrations of ADP and ATP are reciprocally related;
>an accumulation of ADP is accompanied by a decrease in ATP and the amount of energy available to the cell. - Therefore, ADP accumulation signals the need for ATP synthesis.
- ADP allosterically activates isocitrate dehydrogenase, thereby increasing the rate of the citric acid cycle and the production of NADH and FADH2.
- The elevated levels of these reduced coenzymes, in turn, increase the rate of electron transport and ATP synthesis.


