Sulfa, Quinolones Etc Flashcards
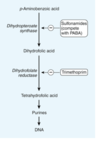
Sulfonamides MOA
block various steps in the purine metabolism pathway, thus inhibiting the production of nucleic acids and DNA
Significance for PABA in bacteria
Many bacteria, unlike mammals, cannot use folate from their environment but must synthesize it from PABA
Relationship between PABA and sulfonamides
As structural analogs of PABA, sulfonamides inhibit dihydropteroate synthase and folate production via competitive inhibition.
Combination of a sulfonamide with an inhibitor of dihydrofolate reductase (trimethoprim or pyrimethamine) provides synergistic activity because of sequential inhibition of folate synthesis.
Mechanism of resistance against sulfonamides
Lack of target: some species of bacteria use exogenous folate and do not have this pathway
Mutated enzyme that drug can’t bind
Toxicities of sulfa drugs
These drugs are all contraindicated in newborns and nursing mothers as they displace unconjugated bilirubin from albumin, increasing the risk of kernicterus.
They can all cause bone marrow suppression that may or may not be due to folate underproduction: nutritional vs. immune-mediated BM suppression, megaloblastic anemia
Sulfonamides increase oxidative stress and thus can precipitate hemolytic anemia in G6PD-deficient patients. This will look like a typical hemolytic crisis you should be familiar with from last year: sudden jaundice, dark urine, normocytic anemia, elevated reticulocyte count.
Solubility of the drugs is a major problem, which can result in crystallization in urinary tract when patients become dehydrated and their urine is more concentrated; treatment is to hydrate and give IV bicarbonate as they are more soluble in alkaline fluids
Rashes are tremendously common with sulfa drugs and can range from photosensitivity, annoying-to-fatal skin rashes like Stevens-Johnson syndrome
Sulfa drugs are very allergenic and can cause hypersensitivity reactions that look like autoimmune disorders: arthritis, conjunctivitis, stomatitis, polyarteritis nodosa, psychosis
Since these hypersensitivity drug reactions are not unique to sulfa drugs, the way they will usually be asked about on exams is to have the patient on a sulfa drug, and then a specific question will be asked about diagnosing the reaction rather than the drug itself.
Examples of dihydrofolate reductase inhibitors
Trimethoprim (TMP) and pyrimethamine
Use of dihydrofolate inhibitors
Commonly be complexed to sulfonamides for synergistic and sequential inhibition of the pathway. TMP is used in bacterial disease complexed as TMP/SMZ, and pyrimethamine for protozoal (parasitic) diseases.
Toxicities of dihydrofolate inhibitors and population most susceptible to them
Toxicity for these drugs is similar to the sulfonamides and increased in AIDS patients, who commonly take these drugs daily for prophylaxis or treatment of opportunistic infections.
Folate DNA pathway
MOA of fluroquinolones
Fluoroquinolones inhibit the DNA gyrase enzyme complex.
This complex has multiple subunit enzymes, and the drugs specifically inhibit:
Topoisomerase II, preventing uncoiling of DNA for transcription and replication, and
Topoisomerase IV, preventing separation of duplicated chromosomes into daughter cells.
First line use of fluroquinolones
Earlier generations of FQs have gram - coverage only, later generations add gram + coverage.
Examples and uses of gen 2-4 of fluroquinolones
- Ciprofloxacin–“urinary FQ”, includes Pseudomonas aeruginosa coverage
- Levofloxacin–“respiratory FQ”
- Moxifloxacin–very broad coverage for resistant pathogens in hospitalized patients
How is fluroquinolones resistance achieved
Low-level plasmid-mediated: Qnr chaperone proteins that protect DNA gyrase. These give a permissive effect so that the bacteria can develop higher-level resistance using:
Efflux pumps
Porin size change
Enzyme point mutations to decrease binding affinity: for example, the gyrA gene in gonococcus
Usually class-wide
Pharmacokinetics/dynamics or FQs
Good bioavailability so useful orally
Mineral or cationic medications will block absorption (remember this problem with the tetracyclines?)
Moxifloxacin is excreted in the bile, which makes it a) not a good choice for urinary tract infections and b) useful in patients with renal failure
Black box warning of FQs
musculoskeletal toxicity most established in the medical literature is tendon rupture
Non-black box toxicities of FQs
Qtc interval prolongation with risk of arrhythmia and sudden death is seen also (remember this is a toxicity for the macrolides as well!).
All fluoroquinolones are considered teratogens due to potential effects on growing cartilage.
Metronidazole MOA
Metronidazole is a prodrug that is selectively absorbed by anaerobic bacteria and protozoa.
Once inside the prokaryotic cell, it undergoes a reduction reaction with reduced ferredoxin, creating active drug metabolites that are toxic and cause oxidative damage to pathogen DNA, proteins, etc
Pharmacokinetics/dynamics of metronidazole
Metronidazole can be given many ways and has good bioavailability when taken orally. It undergoes hepatic metabolism and accumulates in liver failure. It also causes a disulfuram-like reaction (puking, wanting to die) when alcohol is ingested while on the drug due to inhibition of the hepatic enzyme acetaldehyde dehydrogenase that is needed to metabolize alcohol
Metronidazole toxicities
Cumulative neurotoxicity can occur with prolonged courses or with repeated dosing for c. difficile colitis; this can manifest as seizures and peripheral neuropathy.
Metronidazole also has an important interaction with warfarin (coumadin) by altering vitamin K-producing flora in gut; this creates a relative vitamin K deficiency over time and can cause Coumadin toxicity with GI or other bleeding and very high INR/PT.
Phenytoin and Phenobarbital cause accumulation of the drug due to inhibition of the metabolic pathway for the drug in the liver.
Metronidazole also causes lithium toxicity in some patients.
MOA of mupirocin
Mupirocin (pseudomonic acid) is an antistaphylococcal topical agent that is actually a product of a pseudomonas bacteria, P. fluorescens. It is classified as topical only because although it is absorbed, it is too rapidly inactivated to have systemic or tissue effect. The drug has a very specific mechanism of action via inactivation of staphylococcal isoleucyl tRNA sythetase.
Use of mupirocin
It has been the initial drug of choice for topical decolonization of MRSA for many years, but high-level resistance via plasmid-encoded new isoleucyl tRNA sythetase has evolved causing complete loss of activity of the drug.
Polymyxcin B and E MOA
These topical agents are cationic detergents that dissolve/disrupt the lipophilic cell membrane as well as bind and inactivate endotoxin
Use of Polymyxcin B and E
gram - activity only.
Systemic use of these agents is toxic, so it is used clinically in antibiotic ointments (including Neosporin) and in eye and ear drops for those infections.
Fidaxomicin MOA and use
Fidaxomicin is a macrolide that is non-absorbed, used exclusively to treat infections inside the gut lumen. It is a fermentation product of a fungus and binds to the sigma factor required for RNA polymerase binding to gene promoter regions.


