Structure of the immune system: lymphoid tissues and organs week 1 Flashcards
(22 cards)
What are the functions of the lymphoid system?
The lymphoid organs are designed to optimize the recognition function of the immune system. Whenever foreign (non-self) structures cross the non-specific barriers that bar entry into the body, it is the job of the lymphoid system to recognize the presence of the invader, and respond in a very specific fashion that will ultimately result in the elimination of that intruder. The specificity of the response is essential, since the weapons (i.e. effector mechanisms) that can be directed against the foreign antigens.
are highly potent and quite non-selective.
What is the function of primary lymphoid organs?
What are the primary lymphoid organs in humans?
Where are the secondary lymphoid organs?
Primary lymphoid organs: The primary lymphoid organs are the anatomical locations in which lymphocytes develop immunecompetence - the ability to specifically recognize foreign antigen, and (just as important) become tolerant (specifically unresponsive) to self structures. These organs are not sites where foreign antigen is directed to the immune system. One of the most basic concepts of immunology is that the ability to distinguish “self” from “non-self” is established prior to contact with the foreign antigens.
In humans, the primary lymphoid organs are the thymus and the bone marrow.
Peripheral or Secondary Lymphoid Organs All of others, where lymphocyte immune responses occur: includes lymph nodes, spleen, MALT, GALT, tonsils, etc.

What cytokines play an essential role in the early developmental stages of hematopoietic stem cells in bone marrow?
(just list)
CXCL12, IL-7 and IL-3 required
What cells produce IL-7? What hematopoietic lineage is this cytokine especially important for the development of?
What lineage(s) is IL-3 important for the development of?
IL-7 is produced by stromal cells in the bone marrow and is especially important in lymphoid development. IL-3 and a variety of colony-stimulating factors are also essential for myeloid development (granulocytes, monocytes). (IL-3 is responsible for stimulating the differentiation of CFU-GEMM from pluripotent stem cells-see slide 34 of blood and hematopoiesis lecture)
Explain the concept of negative selection for B-cells.
B-cells that have a strong recognition for self antigens in bone marrow are negatively selected and undergo apoptosis. The remaining B-cells express antigen receptors (that are not specific for self antigens) and are released to peripheral lymphoid tissue.

Where do NK cells mature (gain immunocompetence)?
NK cells mature in the bone marrow, lymph nodes and tonsils.

Where do T-cells differentiate? Where do they originate?
The lymphocytes of the B cell lineage leave the bone marrow as immunocompetent cells, capable of recognizing and responding to antigen. They then migrate to the secondary (peripheral) lymphoid organs, where they await the arrival of their predetermined foreign antigen. However, another subset of lymphocytes exits in the bone marrow at a still-undeveloped stage. These cells require further differentiation in the thymus to mature into functional antigen recognizing cells and are therefore called T cells. T cells differentiate along an entirely different pathway from the B cells, recognize antigen only when it is presented in physical association with a self molecule (encoded by the genes of the major histocompatibility complex - MHC), and do not synthesize antibody.
Explain the divisions of the thymus.
What is a unique identfiable structure in the thymus?
How do T-cell precursors get to the thymus? Where do they go within the thymus?
Initmate contact with what cells within the thymus is essential for T-cell maturation?
Where is the blood-thymic barrier located (in what part of the thymus)? What is its function?
Thymic Structure.
The thymus is a lobed organ divided into lobules, with an outer cortex and an inner medulla. The T cell precursors from the bone marrow are transported into the thymus via the blood, migrate to the cortex, and as they mature, they move deeper into the medulla. Intimate contact with thymic epithelial cells, macrophages, and dendritic (interdigitating) cells is essential in T cell maturation. It has special identifiable structures, Hassall’s corpuscles, (concentric layers of epithelial cells of unknown function.
The thymic cortex has blood-thymic barrier. This is due to the structure of cortical capillaries. These capillaries are joined by tight junctions and are ensheated by epithelial reticular cells. This barrier system isolates the developing thymocytes from circulating antigens.

What are the 2 functions of the thymus as it pertains to T-cell development? (just list)
- secretion of thymic hormones
- education
Explain the role of thymic hormones in T-cell development.
Secretion of thymic hormones: There have been at least 5 different thymic hormones identified. These molecules appear to direct T cell differentiation, at least in part. However, they play no direct role in the selection process carried out in the thymus.
Explain the process of education of T-cells. Be specific about the processes and cells involved.
Where in the thymus does education of T-cells take place?
What is the approximate percentage of T-cells that successfully complete the education process?
The thymic epithelial cells, dendritic cells, and macrophages present self antigen (but not foreign antigen) to the developing T cells in the cortex. Those that recognize self MHC molecules sufficiently well will get a positive selection signal to continue dividing, and will establish a clone that will eventually mature in the medulla, followed by exit into the periphery, where they will populate the secondary lymphoid organs. The T cells that fail to effectively recognize self MHC molecules will not receive this signal and will die in the thymus (Failure of positive selection).
But WOE to those T cells that express antigen receptors that bind too well to self MHC molecules! If allowed to leave the thymus, such cells would recognize, and possibly be activated by, self antigens. Thus they would have have the potential to cause autoimmune disease. To prevent this, the thymus gently induces these autoreactive T-cells to commit suicide. This process is called negative selection.
In the end, only about 1% of the T cell precursors in the thymus ever learn the self-recognition lesson correctly, and are allowed to leave, migrating to the secondary lymphoid organs.

What is the blood thymic barrier composed of?
In order to isolate developing T-lymphocytes from circulating antigens there is a Blood-Thymus barrier in the cortex of the thymus. It is composed of the endothelial cells and their basal lamina and the stromal epithelial cells and their basal lamina and a layer of connective tissue between the two.

What are the general functions of secondary lymphoid organs?
Where do B-lymphocytes become plasma cells?
What type of secondary lymphoid organs have afferent vessels? Efferent vessels?
Peripheral (Secondary) lymphoid organs
General functions:
- provide an optimal environment for antigen encounter
- collect populations of antigen specific lymphocytes into organ systems
- drain major routes of antigen entry
- assure maximal encounter with antigen
At these sites, upon reencounter of antigen, B- lymphocytes can become plasma cells producing immunoglobulins
Lymph vessels- only lymph nodes have afferent lymphatic vessels (lymph is conducted to the node). All other peripheral secondary lymphoid organs including the lymph node itself have efferent lymphatic vessels (lymph flows out of the organ).
Where are lymphoid nodules/follicles found in the body?
What are primary follicles?
What are secondary follicles?
Lymphoid nodules or follicles: These are a common feature of all peripheral lymphoid organs. Lymhoid nodules are also found beneath epithelial surfaces throughout the body.
A primary nodule is a follicle which lacks a germinal center.
A secondary nodule is a follicle which has a germinal center. A germinal center appears lighter in staining; it is a center of proliferation of lymphocytes. Presence of germinal centers is a reflection of antigenic stimulation.
Lymphoid nodules are found in:
LYMPH NODES
SPLEEN
APPENDIX
PEYER’S PATCHES
TONSILS
MUCOSAL ASSOCIATED LYMPHOID TISSUE (MALT)
–Includes both Gut Associated Lymphoid Tissue (GALT) and
Bronchus Associated Lymphoid Tissue (BALT)
Describe the lymph circulation.
In what part of the lymph node do afferent vesels enter? What part of the lymph node do efferent lymph vessels exit?
In what part of lymph nodes are B-cells found? T-cells?
What is the function of macrophages and dendritic cells in lymph nodes?
What encapsulates lymph nodes?
Lymph Node Structure: The lymph nodes are the collecting points clustered in specific anatomic locations. They exist in an open circulatory network that allows lymphocytes to systematically monitor the body’s extracellular fluids for the presence of foreign material. The lymph fluid that bathes the cells of the body is collected into the afferent lymphatic vessels which bring antigen into the lymph node. Afferent lymph vessels are a feature unique to the lymph node; its implication is that lymph is conducted to the node. Numerous afferent lymph vessels pierce the capsule at the convex side (subcapsular sinus, see histo lab guid).
Efferent lymph vessels conduct lymph away from the lymph node. These exit the lymph node at the hilum (at concave side) of the lymph node. An extensive system of lymphatic sinuses lies between the afferent lymphatic vessels and the efferent lymphatic vessels of the lymph node. The lymph node:Is completely encapsulated (capsule of dense regular connective tissue).
Immunocompetent B cells are found mainly in the follicles (in the cortex) of the lymph node. Mature T cells are found primarily in the paracortex, which is called the T-dependent region of the lymph node. Macrophages and dendritic cells are found scattered throughout the node, and serve as the initial, non-specific traps for antigen as it enters from the afferent lymphatics.

What is contained within the medulla of lymph nodes?
What is the stroma of lymph nodes?

Medulla consists of:
- medullary cords of cells
- medullary sinsues (lymph sinuses)
Stroma:
- reticular cells
- reticular fibers (type III collagen)
Explain the dual circulation of the lymph node.
What enters the lymph node via each route of circulation?
Receives lymphocytes via afferent lymphatic vessels (also antigen from tissue is made accessible through this route).
Receives lymphocytes via arterial blood which then enter into the node through the postcapillary venules (circulating antigen is also made accessible through this route)

Stimulation of B-cells by antigen induces their terminal differenitation into what cell type? Where in lymph nodes do these cells reside? Describe the mobility of these cells.
Stimulation of B cells by antigen induces their terminal differentiation into plasma cells, which are the main antibody-secreting cells of the body. Plasma cells migrate to the medulla of the lymph node, where they spend the rest of their lives secreting antibody in prodigious amounts.
Explain the mobility of memory cells.
Describe the function of memory cells and the receptors involved in their function.
Another function of lymph nodes is the establishment of immunological memory. Some of the antigen-stimulated B and T cells differentiate into memory cells, which provide the host with long-lived memory for the antigen. Unlike the plasma cells, memory cells can leave the lymph node, exiting via the efferent lymphatic vessels. They eventually find their way to the blood stream (via, for example, the thoracic duct), from where they can spread to other secondary lymphoid tissues. This recirculation of lymphocytes is accomplished by homing receptors on the cells. When lymphocytes in the blood pass through the capillaries of another lymphoid tissue, they encounter specialized structures called the high endothelial venules (HEV), which express surface ligands to which the homing receptors will bind. The lymphocytes then can selectively leave the circulation to enter the secondary lymphoid organ. In this way, stimulation of lymphocytes in one lymph node results in the dispersion of memory cells specific for an antigen throughout the body.
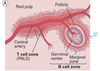
What are the functions of the spleen?
Where in the spleen are B and T-cells located?
Spleen Function
While the lymph node network samples antigens in teh extracellular fluid compartments of the body, the spleen carries out the same function for antigen in the blood. As with the lymph node, the spleen has B cell-dependent areas (follicles, or marginal zone) and T cell-dependent areas (the periarteriolar lymphoid sheath, PALS). During antigenic stimulation, the follicles develop germinal centers, just as in the lymph node.
Filtration of blood: All of the blood is filtered through the spleen. The spleen is to blood as the lymph node is to lymph.
Destruction of senescent red blood cells: The spleen is an important route for removal of senescent red blood cells.
What does white pulp in the spleen consist of?
Where is the red pulp of the spleen in relation to the red pulp? What are the functions of the red pulp?
Where is the marginal zone? What is contained within the marginal zone? (other than B-cells)
What does the red pulp proper consist of?
White pulp
- Contains both T and B lymphocytes
- Consists of:
- a sheath of lymphocytes known as the periarteriolar sheath which is populated by T- lymphocytes.
- primary and secondary lymphoid nodules or follicles distributed at intervals along the length of the central arteriole, which are predominantly B cells.
- Consists of:
Red pulp
- The red pulp of the spleen surrounds the white pulp. An important function of the red pulp is filtration of blood (see red pulp proper, below). Additionally, the red pulp is involved in immune function. The red pulp consists of:
- Marginal Zone- this zone immediately surrounds the white pulp and is an important region for exposure to antigen. The Marginal Zone contains cells essential to the necessary cellular interactions with antigen (macrophage, Antigen Presenting Cells, B and T lymphocytes, plasma cells, etc.).
-
Red pulp proper- consists of:
- Cords (of Billroth)- These are cords of cells which include plasma cells, macrophages, reticular cells and fibers, etc.
- Venous sinuses. These are the sites for filtration of blood. The venous sinuses of the red pulp are channels with wide lumina. They are surrounded by reticular fibers. Their structure is similar to discontinuous capillaries; that is they are lined by special types of endothelial cells with gaps between them.
see slides 37-39 of PP, pg 83 of course notes
What is Mucosa-Associated Lymphoid Tissue (MALT)? Where are they located?
What is the main function of MALT? Collectively, what is the immune system called for all tissues in the MALT system?
Name and describe the MALT within the body.
Where is diffuse lyphocytic filtration found within the body?
There are organized collections of lymphoid tissue scattered throughout the sub mucosa and lamina propria. These are called the Mucosa-Associated Lymphoid Tissue (MALT). They are particularly prominent in the gut where they include the Peyer’s Patches, and in the bronchioles. The main function of the MALT is to sample antigen that enters the host through the mucosa, sites of constant antigenic exposure. Together, the cells of these tissues make up the secretory immune system, which provides a unique and independently regulated protective immune barrier to invasion.
The Appendix: The appendix contains numerous lymphoid follicles (or lymphoid nodules) in its lamina propria and/or submucosa.
Peyer’s patches of ileum: confluent lymphatic nodules in the lamina propria and submucosa of the ileum covered by M (Microfold) cells which transport antigen to underlying lymphoid cells.
Tonsils- The tonsils consist of several tonsillar groups associated with the oropharyngeal mucosa. These are strategically positioned at the opening into the nasopharyngeal and gastrointestinal tracts.
- Lingual tonsils consist of many lymphatic nodules underlying the mucosa at the posterior dorsum of the tongue.
- Palatine tonsils are paired and located on either side of the pharynx.
- Pharyngeal tonsil (adenoids) constitutes a patch of tissue located in the roof of pharynx. same as mucosa of the mouth.
Diffuse lymphocytic infiltation: Lymphoid cells are found in connective tissues throughout the body, in particular in the connective tissue beneath the epithelial lining of digestive, respiratory and urogenital organs.


