SECTION A Flashcards
(30 cards)
What are the basic assumptions of VSEPR theory?
Two main assumptions:
i. Electrons in bonds and lone pairs can be seen as charge clouds that repel each other. Thus, the lowest energy arrangement is when these charge clouds are as far apart as possible, and this determines the equilibrium molecular shape.
ii. Electrons in lone pairs repel more than electrons in bonding pairs and give rise to bent molecules, i.e molecules in which the angles between bonds are different than in molecules with tetrahedral geometry.

State the hybridisation of the C atom in the following molecules:
i. CH4
ii. CH2=CH2
iii. CH(triple bond)CH
i. sp3
ii. sp2
iii. sp
What is the dipole of a bond?
When two atoms with different electronegativity are in a bond, the electronic charge is not evenly spread across the bond → two poles are formed, one with negative electron density and one with positive electron density.
What is a dipole?
A dipole exists when there are areas of asymmetric negative and positive charges in a molecule. The bond dipole moment uses the idea of electric dipole moment to measure a chemical bond’s polarity within a molecule.
What role does symmetry play in the formation of dipoles?
A dipole moment can be seen as a vectorial property. If a molecule is symmetrical, i.e CO2, the dipole vectors cancel out and it has no overall permanent dipole moment.
In H2O, the molecule is bent because of the lone pair on O, thus the two dipoles don’t cancel out and the molecule has an overall permanent dipole moment.
Same as H2O for SO2.
How does the polarity of different molecules relate to their boiling points?
The more polarised the molecule is, the stronger and more numerous the intermolecular forces, the more energy is required to break these intermolecular interactions, i.e more energy has to be put into the system as heat to bring it to boil, the higher the boiling point.
What can the electronic configuration of different atoms tell us about the trend in their boiling points?
Example: Cl2 and Br2
Cl2: 1s2 2s2 2p6 3s2 3p5
Br2: 1s2 2s2 2p6 3s2 3p6 4s1 3d10 4p5
Br has more electrons in outer shells, these are more shielded and experience less effective nuclear charge, as a consequence they are more polarisable.
More polarisable = more intermolecular forces = more energy needed as heat to break these interactions = higher boiling point.
How does the dielectric constant relate to the dipole moment?
Dielectric constants are measured experimental values that determine how well a material stores electrical energy when exposed to an electric field. Generally more polar molecules have higher dielectric constants as they move in response to a field so that their negative and positive ends are correctly aligned.
While dipole moment is a property of an individual molecule, the dielectric constant is a property of the bulk substance (e.g. the liquid).
Higher dipole moments generally lead to high dielectric constants.
How to determine miscibility of two substances?
Like dissolves like:
Polar dissolves polar, non-polar dissolves non-polar.
What is an amphiphile?
An amphiphile is a molecule that has both a hydrophobic and a hydrophilic part, e.g phospholipids found in biological membranes.
What drives the self-assembly of amphiphiles in water?
Hydrophobic effect: amphiphiles self-assemble to minimise entropically unfavourable interactions with water.
What is the basis of the Lewis model of covalent bonding?
Electron sharing between the valence shells of two atoms:
2 electrons = single bond
4 electrons = double bond
6 electrons = triple bond
What is the Pauli exclusion principle?
No two electrons in an atom can have the same four quantum numbers.
What are the four quantum numbers that describe an electron in an atom?
n: principal quantum number. Describes distance from the nucleus. 1,2,3,4…
l: angular momentum quantum number. Describes shape of orbital. Ranges from 0 to (n-1). 0=s, 1=p, 2=d,…
m: magnetic quantum number. Describes orientation of the orbital. Ranges from -l to +l.
ms: spin quantum number. +1/2 or -1/2
What is Hund’s rule of maximum multiplicity?
If two or more orbitals have the same energy, the electrons will spread out to occupy the maximum possible number of these, maximising the number of parallel spins so as to minimise electrostastic repulsion, as this is a lower energy arrangement.
Electrons in different orbitals: less electrostatic repulsion
Electrons with paraller spin: minimise repulsion (due to spin correlation, if they have parallel spins they will stay further away form each other)
This constitutes a lower energy arrangement
What is the formula for bond order?
Bond order = (nº bonding electrons - nº antibonding electrons)/2
Bond order 1: single bond
2: double bond
etc
What are H-bond donors and acceptors?
H-bond donor: H attached to an electronegative atom.
H-bond acceptor: electronegative atom that has a lone pair of electrons (e.g N or O).
Why are H-bonds more short-ranged and stronger than other non-covalent interactions?
The reason for this is that the formation of a H-bond involves overlap between atomic orbitals of the donor and the acceptor.
What is the Grotthuss mechanism?
The Grotthuss mechanism explains why H+ conduction in water is faster than the conduction of similarly charged ions:
H-bonds become covalent bonds, and viceversa, thus causing the transfer of charge from one location to another in aqueous solution without diffusion of any atoms through the water.
How does TLC discriminate betwen H-bonding and non-H-bonding analytes?
Surface of stationary phase displays free Si-O-H groups. H-bonding analytes bind strongly to the stationary phase and thus adsorb to it sooner → analyte is observed near starting point.
Non-H-bonding analytes don’t interact strongly with the stationary phase and thus adsorb further away from the starting point.
What are hypervalent compounds?
Compounds which require more than an octet of electrons in order to draw a Lewis structure are called hypervalent compounds.
In VSEPR theory, it is assumed that if a lone pair has a choice between an equatorial position and an axial position, it will occupy the equatorial site. Why is this?
This is because in the equatorial position it is repelled less by the two axial bonding pairs than it would be by the three equatorial bonding pairs if it was in the axial position.
What is the M.O diagram for O2?
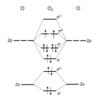
What is the M.O diagram for N2? Why is it different?
The changing in ordering of the MOs can be traced to the increasing separation of the 2s and 2p orbitals as we traverse the period.




