RSNA Virtual Physics School Flashcards
Schematics of Atom
A nuclide can be characterized by
Its mass number A, atomic number Z or neutron number N. Nuclides that have the same Z but different N are called isotopes.
For instance, 125I, 127I, and 131I are isotopes of the same element iodine that contains different numbers of neutrons.
Isobars
Nuclides with the same A are isobars, for example, 131I, 131Xe, and 131Cs are isobars.
They have different N and Z. Due to the different Z, isobars belong to different elements.
Isotones
Nuclides with the same N but different Z are called isotones. 131I, 132Xe, and 133Cs are isotones and have the same N = 78.
Isomers
Nuclides that have the same composition (same N and Z) but carry different energies are called isomers. For example, 99Tc and its metastable state 99mTc are isomers
Characteristic X Ray
When a vacancy in an inner shell is created, an electron in an outer shell promptly jumps in to fill the vacancy. Energy released in this process is equal to the difference in binding energies between the two shells.
The energy may appear as an x-ray photon. Since the binding energies have exact characteristic values, the emitted x-rays carry exact and discrete energies and are called characteristic x-rays.
Auger electron.
Alternatively the released energy may be imparted directly to another orbital electron and makes it free if the energy is enough to overcome the binding energy of the electron This free electron is called Auger electron. No x-rays are emitted in this process.
Both characteristic x-rays and Auger electrons can be emitted by all elements. We cannot predict which process occurs for individual atoms, but can measure the probability of the process for a large quantity of atoms. The measurements indicate that heavy elements are more likely to emit x-rays while light elements more likely to emit Auger electrons.
radioactive decay obeys all conservation laws
mass-energy conservation, electric charge conservation and momentum conservation
y Decay (Isomeric Transition)
In this process, a nucleus in its excited state is transformed to a more stable, lower energy state by emitting a γ photon that carries the excessive energy away.
No changes in the composition of the nucleus occur. Since the transition between two nuclear energy states releases exact amount of energy, the γ photon carries a definite energy. For example, in 99mTc → 99Tc + γ, the energy of γ photons is exactly 140.5 keV.
Both γ decay and internal conversion achieve the same goal, that is, to release excessive energy of the nucleus. For individual nuclei, we cannot predict which decay may occur. Instead, the probabilities of the decays are measured for a large quantity of nuclei.
Internal Conversion
As an alternative to γ decay, a nucleus may release energy by imparting it directly to an orbital electron and ejecting it from the atom if the energy is sufficient to overcome the binding energy of the electron. The energy excess above the binding energy becomes the kinetic energy of the conversion electron. The orbital electrons in the K or L shell are most likely to be ejected since they are closest to the nucleus. This nuclear decay is one example that involves orbital electrons.
The orbital vacancy left by the conversion electron is subsequently filled by an outer-shell electron. This transition results in emission of characteristic x-rays or Auger electrons as discussed above.
β- Decay
In this process, a neutron in the nucleus changes to a proton plus an electron and an antineutrino, n → p + e- +
The antineutrino is the antiparticle of a neutrino that carries energy but neither mass nor charge. The electron and antineutrino are created within the nucleus and are ejected out of the nucleus right away.
Therefore the net effect of the decay is the transformation of a neutron to a proton .
Therefore this decay most likely occurs for an unstable neutron-rich nucleus. By decreasing N and increasing Z, N/Z of the child nucleus decreases to a more stable value.
The mass number A remains same because the total number of nucleons does not change
β+ Decay and Annihilation
A proton in the nucleus is transformed to a neutron, a positron (e+) and a neutrino (n) in the process: p → n + e+ + v.
The positron is the antiparticle of an electron so it is exactly the same as an electron except that it carries positive electric charge. The positron and neutrino are created in the nucleus and are ejected out of the nucleus instantaneously so the net effect is that a proton becomes a neutron in the child nucleus.
After a positron is emitted from the nucleus, it loses its kinetic energy in collisions with surrounding electrons and comes to almost rest within a few millimeters. At this moment, the positron combines with an electron. Both particles disappear and two new photons are created, e+ + e- → 2γ.
PET imaging
The positron emission and successive annihilation are the physical foundation of PET imaging.
The most often used PET radionuclide, 18F, decays to 18O:
18F → 18O + e+ + n. The annihilation photons are then detected coincidently using a PET scanner to form the patient’s image.
Electron Capture (EC)
In this process, an orbital electron in an inner shell is “captured” by the nucleus
Once it enters the nucleus, the electron immediately combines with a proton to form a neutron: p + e- → n + ν. The neutrino is then ejected from the nucleus.
As a result, the child nucleus has one more neutron and one fewer proton, that is, the net effect of EC . Thus EC and β+ decay have the same effect
Decay and Nuclear Fission
In α decay, the nucleus ejects an α particle that consists of two protons and two neutrons (actually a 4He nucleus)
In nuclear fission, a heavy nucleus spontaneously breaks into two lighter nuclei. Two or three neutrons are emitted and also huge energy (~ hundreds of MeV per fission) is released. The energy can be used to generate electricity or for atomic weapons.
Both decay modes occur among heavy nuclides that are not directly used in nuclear medicine
Secular Equilibrium
The first situation applies when Tp is so long (“almost infinite”) that the decay of the parent is negligible. The parent activity Ap shown by the red line almost does not change with time whereas the child activity shown by the blue line increases initially and after about five child half-lives, it equals the parent activity, Ac = Ap. When this occurs, the parent and child are in secular equilibrium. An example is 226Ra (Tp = 1620 y) → 222Rn (Tc = 4.8 d) → … → 210Pb.
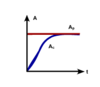
Transient Equilibrium
If the parent half-life is longer than the child half-life but is not that long, the parent and child may be in transient equilibrium. The parent activity decreases with time as shown by the red line in Figure.
The child activity shown by the blue line increases initially and eventually exceeds the parent activity to reach its maximum. After that, the child activity decreases following the decay of the parent, that is, the ratio of parent-to-child activities keeps constant with time. For this stage, the parent and child are in transient equilibrium.
The ratio of the parent-to-child activities and the time at which the child activity reaches its maximum are determined by the half-lives of the parent and child. In the chain decay of
99Mo (Tp = 66 h) → 99mTc (Tc = 6 h) → 99Tc,
the peak time is about 24 hours. That is, we should extract 99mTc activity from the generator once a day at the same time. If the opportunity is missed, the 99mTc activity decreases from its peak value so the activity is wasted.

No Equilibrium
When the half-life of the child is longer than that of the parent, there is no equilibrium between them as shown in Figure.
One example is β- decay of 131mTe:
131mTe (Tp = 30 h) → 131I (8 d) → 131Xe.
The child activity shown in blue increases initially with time, reaches a maximum, and then decreases. When the parent activity decreases to zero, the remaining child activity continuously decreases with its own half-life.



