Renal Phys: Body Fluid comp PART 2 Flashcards
Roughly, what are the water % of men, women, children and obese people?
Varies with sex, age and body fat. The more fat: women/ obese the less water you have. ~50-70%

Where is the 50-70% water in our bodies??
Two Compartments: Total water 42L
- INSIDE CELLS (intracellular fluid; ICF) 2/3 or 28L
- OUTSIDE CELLS (extracellualr fluid; ECF) 1/3 or14L
A. Interstitual fluid (ISF) 11L
B. Plasma 3L
How does the water move between compartments?
- All the membranes in the body are permeable to water (exception: kidneys, ureters and bladder that have tigher membranes). Therefore water has a ‘backstage pass’ and can move anywhere in the body, following certain gradients.
- Water flows from a low to high conc of osmotically active molecules.
Osmolarity vs Omolality
Osmolality: number of osmotically active particles per unit weight of solvent. Units: milli Osmoles/kg of solvent.
This determines the osmotic pressure exerted by a solution across a membrane.
Osmolarity: the number of osmotically active particles per litre of total solution. Units: mOsmol/L
What is Tonicity and why does it get confused with osmolality?
Describes the osmotic pressure a solute exerts across a membrane (thereby causing movement of water).
Only accounts for osmotically active impermeable solutes.
Not readily measurable and unlike osmolality is in reference to a particular membrane.r

The plasma membrane of cells is a semi-permeable membrane: permeable to water but NOT charged molecules. So what do the terms hypotonnic, isotonic and hypertonic mean?
- Hypotonic: makes cells swell, water moves into cells
- Isotonic: cells stay the same size, water is in/out at an equal rate
- Hypertonic: makes cells shrink (water moves out of the cell)
Cells are full of proteins that are ____________ but ____________ to the membrane.
Cells are full of proteins that are osmotically active but impermeable to the membrane.
Charged particles seperated by a semi permeable membrane can fail to evenly distribute evenly in the presense of a non-diffusible ion. What happens now?
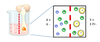
Negative charges want to move down their conc gradient.
Then Positive ions want to follow to balance charges, but then they wanna go back don their concentration membrane.
Competing electrical and conc gradients means that at equilibrium the side with the proteins is more negatively charged
= ‘voltage gradient’

What is the consequence of the voltage gradient?
More osmotically active molecules are on the protein side (greater osmolality) therefore water flow into the protein side (‘oncotic pressure’). Cells need to balance this or they’d pop!

Whats the bodies solution to balancing osmostic pressure across the membrane (so cell doesn’t burst).
Solution: pump out osmotically active ions (Na+) using the Na+/K+ ATPase transporter
Net result: K+ and proteins inside balance Na+ outside. Interstitual fluid and ICF are isotonic.
How are these all the same osmolality?

Because despite differences in electrolyte composition they overall balance out to ~ 300mOsm/Kg

Normal ECF osmolality
ECF osmolality is dominated by Na+ (due to Na+/K+ ATPase)
~300mOsmol/kg
Controlling ECF osmolality is CRITICAL for cell survival and only varies 1-2%, regulated by altering water levels.
ECF osmolality - Hypotonic
Solution to this.
Will cause cells to swell via osmosis, brain swells ( ouch!) can cause death.
eg: if you rink too much water > brain swells > dies
Solution: Body removes excess water to concentrate Na+ via kidneys.
ECF osmolality - Hypertonic
Solution to this.
Hypertonic ECF will cause cells to shrink by osmosis > brain shrinks (ouch!) can lead to death.
Solution: retains fluid to dilute Na+
ECF volume
- Less tightly controlled (regulated within range of 15%)
- The volume of the ECF depend primarily of the amount of Na+ (major osmo active solute in the ECF)

The major regulator of water and salt homeostasis (ECF osmolality and volume)
The Kidney: like a tap that turns on and off
**Starlings Forces: also important for governing movement of fluid across compartments.
Example of starlings forces: Oedema
- Abnormal expansion of the interstitual fluid compartment (can be localised or general)
- Results from changes in Starlings forces in the plasma leading to movement of fluid into interstitual space
Causes: inflammation, lymp/venous obstruction, sodium retention, low serum albumin.
Urine output and urine osmolality varies to balance
water and salt levels


