Renal Control Flashcards
what is pH a measure of?
it is a measure of the acidity or alkalinity of a solution
pH= -log10[H+]
what is a buffer?
a compound that resists changes in pH
what range of pH does the blood stay in ?
7.35-7.45
what organ maintains the blood pH?
lungs and kidneys
why is it necessary to maintain plasma pH constant?
to maintain the pH of cell fluid at approximately ph 7.0 which is necessary for cellular reactions to occur
metabolism of carbohydrates and fats produces what?
produces CO2 and H2O (CO2 is acidic and is eliminated by the lungs)
amino acid metabolism produces what?
Cystein and Methionine (sulfur containint) produce sulfuric acid
Lys, Arg, and His AA prduce HCl
partially offset by Asp, Glut, which produces alkiline produce
metabolism of organic anions produces what?
eX) citrate - produces Alkali (HCO3-)
What factors effect blood pH?
metabolism of carbohydrates, fats, amino acids, organic anions, and dietary components
*in general net acid production exceeds alkali*
which buffer system (unlike Phosphate buffer) is regulated by both the lungs and the kindey?
HCO3- (bicarbonate) buffer system

what equation quantifies how changes in CO2 and HCO3- affect pH?
Henderson - Hasselbach Equation
the pH of the system depends on the ratio of molar conc. bicarbonate to pressure carbon dioxide

[HCO3] is maintained at what constant molar volume by the kidneys?
24mmol/:L
paCO2 is maintained at what constant pressure by the lungs?
40mmHg
is there a tendency for plasma [HCO3] or paCO2 to change and consequently alter the pH?
Yes.
there is a tendency for pCO2 to rise and for [HCO3] to fall - either would result in a decreased pH
normally the pH does not decrease because the lungs and kidneys counteract these changes and prevent the change in pH
where is CO2 produced?
it is continuously produced by cellular metabolism in all tissues - the pressure of CO2 does not rise in the blood and lower the pH because the lungs eliminate CO2 as rapidly as it is produced
how does the bicarbonate concentration of the blood fall? [HCO3]
it is a buffer for dietary components of acid or end products of metabolism - these acids are buffered by bicarbonate thus lowering the concentration of bicarb
how does the kidney prevent a decrease in plasma [HCO3] and a fall in pH?
- tubular cells reabsorb all the [HCO3] from the tubular filtrate - maintains the plasma [HCO3-] at its existing level
- the tubular cells synthesise an amount of bicarb equivalent to that consumed in buffering and add it to the blood - this will restore plama [HCO3] to normal

describe how HCO3 is reabsorbed by intercalated cells of collecting duct

describe how HCO3 is secreted by beta intercalated cells of collecting duct
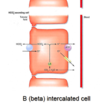
how is generation of new HCO3- achieved?
- by the excretion of H+ with non HCO3- urinary buffers
- by the synthesis and excretion of NH4+ (non-ionic diffusion and diffusion trapping) - most of the NH4+ formed in the PT is reabsorbed in the TAL with NH4+ substituting for K+ on the Na/K/2Cl symporter



