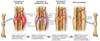
Regeneration and Repair Flashcards
What are the processes involved in wound healing?
- Haemostasis
- stopping the flow of the blood as vessel are open
- Inflammation
- injury to the tissue triggers the inflammatory response
- Regeneration and/or Repair
- structures have been injured or destroyed and need to be repaired
What is regeneration?
The resolution or restitution of an injury to replace structures that have been lost or damaged. There should be little or no evidence of previous injury. Examples include healing by primary intention (the closing of a wound) or the regeneration of a superficial abrasion.
What is the difference between an abrasion and an ulcer?
In an abrasion the damage is very superficial and does not damage the skin further than the mucosa. The tissue is able to undergo complete restitutionwithout any intervention
The tissue damage in an ulcer extends into the submucosa and an area of scarring will occur.
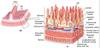
Which cells replicate in regeneration?
Newly differentiated cells are mainly derived from stem cells as many termically differentiated cells cannot divided.
What are stem cells?
Cells with prolonged proliferative activity which show asymmetric replication (one daughter cell remains a stem cell while the other mature).
They express an internal repair system to replace lost or damage cells in tissue (self-renewal).
What is the difference between embryonic stem cells (eSCs) and adult stem cells?
eSCs are pluripotent and can differentiate into any cell of the body where as adult stem cells can normally only give rise to one type of cell (unipotent) of a specific lineage.
Where in the tissue are adult stem cells found?
Varies dependant on tissue:
- Epidermis - basal layer adjacent to the basement membrane
- except from the lens of the eye and renal podocytes
- Intestinal mucosa - bottom of intestinal crypts
- Liver - inbetween hepatocytes and bile ducts
What are the examples of unipotent, multipotent and totipotent stem cells?
Unipotent:
- most adult stem cells that can only produce one type of differentiated cell e.g. epithelia
Multipotent:
- produce several different type of differentiated cell e.g. haemtopoeitic stem cells
Totipotent:
- embryonic stem cells
- can produce any type of cell/tissue in the body
Can all tissues regenerate?
No, only labile and stable tissue. Permenant tissues cannot regenerate.
What are labile tissues?
Continuously dividing tissues that proliferate throughout life, replacing cells that are destroyed.
Examples:
- surface epithelia
- mucosal lining of secretory ducts
- columnar epithelial in GI and uterus
- tranistional epithelial in urinary tract
- bone marrow cells and haematopoetic tissues
What are stable tissues?
‘Quiescent tissues’ - dormant/inactive
Low levels of replication but can undergo rapid division in response to stimuli to reconstruct the tissue of origin.
Examples:
- parenchymal cells of the liver, kidneys and pancreas
- mesenchymal cells
- fibroblasts
- bone osteoclasts
- smooth muscle cells
- vascular endothelial cells
- resting lymphocytes
- other WBC
What are permanent tissues?
Non-dividing mature cells that cannot undergo mitoses in postnatal life and there are no or only a few stem cells present.
Examples:
- neurones
- skeletal muscle cells
- cardiac muscle cells

In what circumstances can regeneration take place?
- when the damage occurs in labile or stable tissue
- the damage in not extensive; there is still an intact connective tissue scaffold
What is fibrous repair?
Healing with formation of fibrous connective tissue froming a scar.
- the specalised tissue is lost
- secondary intention healing (wound is extensive and involves considerable tissue loss
When does fibrous repair/organisation occur?
- when significant tissue is lost
- when permenant of complex tissue is injured
Outline the pathways to regeneration and fibrous repair

How does a scar form? Give timeframe
- Haemostasis (seconds - minutes)
- Acute inflammation (minutes - hours)
- Chronic inflammation (1 - 2 days)
- Granulation tissue forms (3 days)
- Early scar (7 - 10 days)
- Scar maturation (weeks - 2 years)
What is granulation tissue?
Tissues that consists of:
- developing capilleries
- very vascular and red
- fibroblasts and myofibroblasts
- chronic inflammatory cells
It has a granular appearance and texture. New connective tissue that contains microscopic blood vessels as part of the healing process. Grows from the base of the wound and can fill any size.

What is the function of granulation tissue?
- fills the wound gap
- capillaries supply oxygen, nutrients and cells
- contracts and closes the hole
- orchestrated by myofibroblasts
What are the stages of fibrous repair?
- Blood clots
- Neutrophils infiltrate and digest clot
- Macrophages and lymphocytes are recruited
- Vessels sprout. Myofibroblasts and fibroblasts make glycoproteins
- Vascular network formed, collagen is synthesised and macrophages reduce
- Maturation of the wound, cells are reduced, collagen matures and contracts and remodels
- can occur 1-2 years after the injury.