Rate in chemical rxn Flashcards
Rate Law Summary
Rate law - rate (speed) of reaction (in moles / litre / second) can be expressed as function of concentration of REACTANTS (not products!).
Rate law equation in chemical reaction:
rate = k[A]m[B]n
[] = concentration of corresponding reactant in m/L
k = rate constant
m = order of reaction with respect to A
n = order of reaction with respect to B
m + n = overall rate of reaction order
Reaction rate dependent ONLY on concentration of reactants not products!
- Note: Rate constant k is reaction specific, directly proportional to rate of reaction. It increases with increasing temperature since proportion of molecules with energies greater than activation energy Ea of reaction increases with higher temperatures.

Rate Orders
- Zero rate order for reactant A (m = 0) - rate of reaction INDEPENDENT OF CONCENTRATION of reactant A (or B) = constant reaction rate. Rate equation expressed as rate constant k. Rate depends on temperature or other factors excluding concentration.
Rate = k
- First rate order for reactant A (m = 1) - rate of reaction DIRECTLY PROPORTIONAL TO CONCENTRATION of reactant A (or B), rate equation expressed as:
Rate = k[A]1 OR
Rate = k[B]1
- Second rate order for reactant A (m = 2) - rate PROPORTIONAL TO SQUARE OF REACTANT CONCENTRATION, rate equation expressed as:
Rate = k[A]2 OR
Rate = k[B]2
Rate Orders:
Concentration vs Time Diagram
Figure:
- For zero-order reactant - as concentration of reactant A decreases over time, slope of line is constant = rate is constant. Rate doesn’t change regardless of decrease in reactant A concentration over time = zero order rate order.
- For first order reactant - decrease in reactant A concentration affect rate of reaction in direct proportion = concentration decreases = rate decreases proportionally (slope↓)
- For second order reactant - rate of reaction decrease proportionally to square of reactant A concentration (slope↓)
NOTE! Curves for 1st & 2nd order reactions resemble EXPONENTIAL DECAY. 2nd order reactions decay faster.

Rate Order: 0
Straight line Diagram
Reaction Order: 0
Rate Law: Rate = k[A]0
Integrated Rate Law: [A]t = -kt + [A]0
Units of k: M⋅s-1
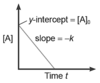
Rate Order: 1
Straight line Diagram
Reaction Order: 1
Rate Law: Rate = k[A]1
Integrated Rate Law: In[A]t = -kt + In[A]0 or ln[A]t/[A]0 = -kt
Units of k: s-1

Rate Order: 2
Straight line Diagram
Reaction Order: 2
Rate Law: Rate = k[A]2
Integrated Rate Law: 1/[A]t = kt + 1/[A]0
Units of k: M-1 ⋅ s-1

Rate Law Summary Diagrams
Half-Life of Reaction
Half-life of reaction – time needed to decrease concentration of reactant to one-half of original starting concentration.
Each rate order has its own respective half-life.
Half life for first order is constant.
Half life for second order is increasing.
Half-Life 1st Order
First order: length of half-life is constant
Half-Life Equation: t1/2 = 0.693/k = 1/k(0.693)

Half-Life 2nd Order
First order: length of half-life is increasing
Half-Life Equation: t1/2 = 1/k[A]0 = 1/k ·1/[A]0

Determining Rate Order From Experiment
First 3 experiments - [A] changes but [B] remains same, resultant changes in rate only depend on concentration of A, when [A] doubles (Exp. 1 & 2) reaction rate doubles, and when [A] triples (Exp. 1 & 3) reaction rate triples = directly proportional, exponent of [A] must be 1 & rate of reaction is first order with respect to A.
Final 3 experiments - [B] changes while [A] remains same, when [B] doubles (Exp. 3 & 4) rate increases by factor of 4 (= 24); when [B] triples (Exp. 3 & 5) rate increases by factor of 9 (= 54) = relation is exponential where exponent of [B] is 2 – rate of reaction is second order with respect to B.
- initial rate = k[A]1 [B]2
- Overall rate of reaction (n+m = 1+2 =3) is 3rd order.

Rate-Determining Step
Rate of overall reaction is naturally limited by slowest step = rate-determining step in mechanism of reaction is slowest step - overall rate law of reaction = rate law of slowest step.
Faster processes have indirect influence on rate à regulate concentrations of reactants & products.
- Chemical equation of each elementary step reflects exact molecular process that transforms its reactants into its products - its rate law can be predicted from its chemical equation - in elementary process, orders with respect to reactants are equal to corresponding stoichiometric coefficients.
If non-elementary reaction (several steps) with slowest step – rate overall = rate of slowest step (if visible within written reaction)
If elementary reactions (single step) – rate = stoichiometry.
Reactants and products have opposite signs but same constant rate.
Dependence of Reaction Rates upon Temperature
Many reactions slow down by ↓T & get faster by ↑T
From collision theory of chemical kinetics: rate constant of reaction: k = A·e(-Ea/RT)
Rate constant Arrhenius equation describes relationship between rate constant (k) & temperature:
- A = Arrhenius constant (frequency factor), includes 2 components: orientation factor (p) & collision frequency (z). Collision frequency (z) – # of collisions that molecules acquire per unit time, orientation factor (p) – proper orientation reactant molecules require for product formation. Arrhenius constant related to both frequency of collisions (z) & proper orientation (p) of molecular collisions required for final product formation: A = pz
- e = base of natural logarithms
- Ea = activation energy – energy required to get reaction started
For reactants to transform into products, reactants must go through high energy state or “transition state” = minimum energy (activation energy) required for reactants to transform into products. If 2 molecules of reactants collide with proper orientation & sufficient energy or force in such way that molecules acquire total energy content surpassing activation energy, Ea, collisions result in complete chemical reaction & formation of products. Note! Only fraction of colliding reactant molecules will have sufficient kinetic energy to exceed activation energy barrier.
- R = ideal gas constant (1.99 cal mol-1 K-1)
- T = absolute temperature, NEVER NEGATIVE!
Shown in equation k = A·e(-Ea/RT) that rate constant, k, contains T component as exponent - T affects reaction rate by affecting actual rate constant k.
- Note: Rate constant remains constant only when T remains constant.
- Either ↑T or ↓Ea will result in ↑constant k = ↑reaction rate. Because -Ea/RT is negative exponent, so biggest value when negative exponent is small, so ↑T or ↓Ea makes exponent smaller.
Arrhenius Equation

Exothermic Reaction
- Total energy of reactants (A+B) is higher than total energy of products (C+D) = exothermic reaction.
- ΔH = negative = exothermic

Endothermic Reaction
- Total energy of reactants (A+B) is lower than total energy of products (C+D) = endothermic reaction.
- ΔH = positive = endothermic

Difference between PE
& activated complex
- Difference in potential energy between reactant(s) & activated complex = activation energy of forward reaction.
- Difference between product(s) & activated complex = activation energy of reverse reaction.
- Note! The bigger the difference between total energy of reactants & activated complex = activation energy Ea the slower the reaction.
Catalyst
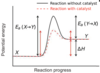
Catalyst - increases rate of chemical reaction without being consumed by reaction.
Catalysts help ONLY by lowering Ea of reaction & help reaction to proceed = ↑Rateinitial.
Enzymes as typical biological catalysts – protein molecules with very large molar masses containing one or more active sites. Enzymes = very specialised catalysts – generally specific & operate only on certain biological reactants called SUBSTRATES.
Enzymes increase rate of reactions by large factors.
REMEMBER: Equilibrium K not affected by catalyst! Catalyst has no effect on K, ΔH, ΔE, ΔG or Q
Reaction with or without catalyst
Potential energy diagram:
showing both exothermic without catalyst & exothermic with a catalyst - forward reaction being endothermic, thus reverse reaction is exothermic.
Saturation Kinetics
Saturation kinetics diagram:
- When concentration of substrate large enough for substrate to occupy all available active sites on enzyme, any further increase would have no effect on rate of reaction = saturation kinetics

Equilibrium constant Keq
Equilibrium constant Keq - relative concentrations of all components of forward & reverse reactions become constant at equilibrium = state of “dynamic equilibrium
aA + bB ⇌ cC + dD
Keq = [C]c[D]d / [A]a[B]b
K value = indication of where equilibrium point of reaction actually lies, either far to right or far to left or somewhere in between.
- If Keq > 1 – forward reaction is favoured = reaction favours product formation. If K is very large, equilibrium mixture will then contain very little reactant compared to product.
- If Keq < 1 – reverse reaction is favoured = reaction doesn’t proceed very far towards product formation and thus very little product formed.
- If Keq = 1 – neither forward nor reverse directions are favoured.
- Note! Pure solids & pure liquids don’t appear in equilibrium constant - heterogeneous equilibria, since liquid & solid phases are not sensitive to pressure, their “concentrations” remain constant throughout reaction = their values denoted as 1.
Law of Mass Action
LAW OF MASS ACTION:
Equilibrium constant K (or Keq) has given value at given temperature:
If T changes value of K changes.
At given T, if we change concentration of A, B, C or D, system evolves in such way to re-establish value of K.
For chemical reaction mixture that is in equilibrium, ratio between concentration of reactants & products is constant.
With chemical equilibria there is constant interplay between molecules but, at given temperature, equilibrium constant Keq remains constant.
Le Chatelier’s Principle
Le Chatelier’s principle -
whenever perturbation/stress applied to system at equilibrium, system evolves to compensate for applied perturbation = relieve the stress.

Le Chatelier’s Principle Diagram



