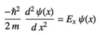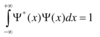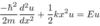Quantum mechanics Flashcards
(30 cards)
Give the Schrödinger equation in its most basic form
H is the Hamiltonian operator
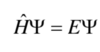
Give the Schrödinger equation for a particle of mass m moving in one direction with energy E

State some phenomena that are explained by quantum mechanics
- Photoelectron effect
- Atomic spectra
Give an equation for kinetic energy involving momentum
T = p2/2m
State the velocity of a free particle
Velocity is constant for a free particle
Give the equation for wavevector

Give the equation for angular momentum

Give the equation for wave velocity

Draw out the first 4 waves if a particle is confined to a box

State the position of nodes for a standing wave

Give and describe Wien’s displacement law
This law gives the observed shifts in the maximum of emission curves
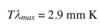
Give and describe the Stephan-Boltzmann law
M is the power emittance, ie rate of energy output

Give and describe the Rayleigh-Jeans law
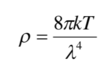
Describe the photoelectric effect and how it is an example of wave-particle duality
- When a metal is exposed to UV light, electrons are ejected from the metal’s surface
- Below a threshold frequency, no electrons are emitted
- Even at low intensities, electrons are emitted as long as threshold frequency is reached
- The kinetic energy of the electrons varies with frequency but not with intensity
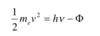
State the de Broglie relationship

Define the Hamiltonian operator
H = T + V
Hamiltonian operator = kinetic emergy operator + potential energy operator
Describe quantum tunnelling
Quantum tunnelling describes the phenomena of a subatomic particle being able to travel through a classically forbidden region. The particle’s behaviour can be explained by it having wavelike properties
V is the potential barrier, E is the energy of the particle, a is the thickness of the barrier in m

Describe Heisenberg’s uncertainty principle
“It is impossible to specify simultaneously with arbitrary precision, both the momentum and the position of a particle”
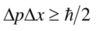
Describe cases in which quantum tunnelling is present in chemistry
- The oxidation and reduction of metals. When ligands are present, the transferred electrons seem to pass through them
- Electrical contacts. Metal surfaces are usually coated with a thin layer of oxide. Conduction can take place by tunnelling through this layer.
- Electrode reactions.
- Photon transfer
- Bond rotation
Describe the scanning tunnelling microscope
The microscope consists of an atomically sharp tip. A voltage is applied between the tip and the surface, ensuring they are very close but not touching so that tunnelling occurs

Give the momentum operator in 1 dimension
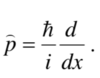
Give the Schrödinger equation for a free particle moving in 1 dimension
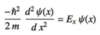
Give examples of a free particle
- A gas molecule in a big container
- An electron conducting in a metal
- An electron beam
Give the Schrödinger equation for a particle in a box
The particle is no longer free, although V inside the box is 0. Outside V is infinite - the particle cannot exist at or outside the walls as infinite energy is impossible.
Note: There are boundary conditions. Ψ at the edges of the box = 0