Programmed cell death Flashcards
(28 cards)
Why is programmed cell death necessaary?
- sculpting structures during development.
- deletion of structures
- controlling cell number (80% oocytes in females deleted)
- eliminating dangerous and abnormal cells (deletion of immune cells)

Major types of programmed cell death
- apoptosis
- necroptosis
- autophagy
Describe apoptosis.
- active process by which cells undergo suicidal death
- cascade of molecular interactions
- induced by external or internal signals
- inflammation and tissue damage not seen (death with dignity)
Describe necroptosis.
- violent death
- depends on rip1 and rip3 kinases
- inflammation and tissue damage can result
What is the morphology of apoptosing cell?
- shrinkage
- membrane blebbing (untethering of membrane)
- phosphatidyl serine exposure on cell surface (normally kept inside by flippases)
- DNA fragmentation and 200bp DNA laddering
- chromatin/nuclear condensation
- nuclear fragmentation
- plasma membrane integrity is preserved
How was it determined that plasma membrane is kept intact during apoptosis?
annexin V - FITC/PI double staining
- propidium iodide: stain DNA red. Intact cell membrane does not allow to enter the cell
- Annexin V: binds to phosphatidyl serine on the cell surface
flow cytommetry shows that when cells are treated with both, cells are only stained with annexin V

How was DNA fragmentation and laddering determined as a result of apoptosis?
- Fixed cells treated with empty vector or with Bax, an inducer of apoptosis.
- staining with TUNEL reveals areas of DNA fragmentation, because dUTP binds free 3’ OH ends, causing fluorescence.
- DNA gel shows ladder fragmentation in the presence of Bax
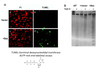
Describe the 3 steps of apoptotic cell removal.
- phagocyte attacted to a “find me” signal
- recognition and phagocytosis via displayed “eat me” signals and lacking “dont eat” signals
- production of anti-inflammatory cytokines
How does phosphatidylcholine function in apoptosis?
It is on the cell surface and acts to attract phagocytes and as an “eat me” signal
List the two ways in which apoptosis can be induced.
- extrinsic: receptor-mediated signaling (e.g. TNF receptor)
- intrinsic: DNA damage, unfolded protein response (ER), growth factor withdrawal (some cells default to death without constant growth factor), oxidative stress
What is the protein responsible for initiating apoptosis? Describe them.
Caspase
- cystein protease with active site QACxG
- cleave after aspartate residue
- present as zymogen until cleaved by another caspase (dangerous to just have floating around in active state)
- initiator caspases
- executioner caspases
- other functions in addition to apoptosis
- involved in inflammation in other pathways.
Describe the domains of initiator caspase
- CARD domain: recruits executioner caspase
- DEAD domain: dead effector
List the initiator and executioner caspases.
initiator: caspases 2, 9, 8, 10
executioner: 3, 6, 7
Describe the activation of procaspase.
Procaspase is cleaved at N and C termini by a different, already activated caspase, releasing prodomains and allowing two small subunits to come together with large subunits on the outside. It is now active.

Describe the caspase cascade.
one molecule of activated initiator caspase can go on to activate many executioner caspases, which will cleave cytosolic proteins and cleave nuclear lamins (both events necessary for apoptosis)

Describe the extrinsic pathway of apoptosis initiation.
- killer lymphocyte such as CD8 presents FAS ligand on its cell surface which binds to FAS death receptor on target cell
- activation of FAS death receptor initiates assembly of DISC (death-inducing signaling complex) consisting of FAD adaptor protein (with death and death effector domains), and procaspase 8/10.
- assembly of DISC activates procaspase, so that it can go on to activate executioner caspases
- activation of executioner caspases results in apoptosis.

Describe the intrinsic pathway leading to apoptosis.
- mitochondria receives apoptotic signal (ROS, stress, ect), causing it to release cytochrome c
- cytochrome c binds and activates Apaf, revealing its CARD domain which is normally hidden
- apoptosome assembles by 7 Apaf proteins polymerizing with CARD domains at the center
- procaspase 9 is recruited and assembles to the CARD domains, causing it to be activated
- caspase 9 then cleaves and activates executioner procaspases, leading to apoptosis.

Describe several of the functions of caspases in addition to apoptosis initiation.
Can play role in tissue remodeling via Wnt signaling, but same pathway can cause therapy resistant tumor cells.
What is the anti-apoptotic protein involved in intrinsic apoptotic signaling?
BCL2
- expressed at high levels in cancer cells because it protects cells from death
- founding member of BCL family of proteins
Describe the 3 types of BCL proteins.
- anti-apoptotic BCL2. Has four domains, BCL homology domains 1, 2, 3, 4
- pro-apoptotic BH123 protein. Has three domains, BCL homology domains 1, 2, 3
- pro-apoptotic BH3-only protein. Only has domain BH3.
How are anti-apoptotic proteins inhibited?
By interaction with pro-apoptotic proteins BH3 and BH123.
Can also be inhibited by small molecule with BH3 mimicking domain.
How is the apoptosis stimulus perceived by the mitochondria?
Apoptotic signal causes BH123 on mito surface to aggregate, forming pore to allow cytochrome c to exit mito. First requires inactivation of anti-apoptotic Bcl2 by an active BH3 protein.

Describe the role of anti-IAP in the intrinsic apoptosis signaling pathway.
- anti-IAP is a protein which lives inside the mitochondria. It binds to an inhibits IAPs, which themselves bind to and inhibit caspases.
- when an apoptotic stimulus arrives at the mito, anti-IAPs are released through BH123 pore and allow for the successful spontaneous activation of initiator and then executioner caspases.

Which signals can enhance the intrinsic apoptosis signaling pathway?
- survival factors (growth factors) can bind a target cell, leading to increased expression of BCL2, which blocks apoptosis.
- survival factor can bind target cell and cause activation of Akt kinase, causing Bad (BH3 protein) to be phosphorylated and inhibited, releasing BCL2 so it can block apoptosis.
- survival factor can activate MAP kinase, phosphorylating and inactivating Hid (an anti-IAP), so IAP can continue inhibiting apoptotic proteins (caspase) to block apoptosis.



