Physiology of Muscle Contraction: Flashcards
(32 cards)
Troponin: how it works
-Troponin, or the troponin complex, is a complex of three regulatory proteins (troponin C, troponin I, and troponin T) that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle
-Troponin has 4 calcium-binding sites.
- 4 Ca2+ bind to the troponin C in skeletal muscles (C = calcium binding) site of the troponin
- In the heart, Troponin C only binds to 3 Ca2+ ions
- when Troponin C binds to calcium, it changes conformation which turns off troponin I.
- conformational change in Troponin C drags the tropomyosin out of the binding site of the myosin-binding site so that myosin and actin can interact
- myosin head then binds to actin, making cross bridges which is what causes the forced generation due to ATP consumption.
- Myosin will break down ATP and myosin pull the thin filament by grabbing the two actin filaments from opposite sites towards one another
- Troponin I is a marker for muscle breakdown. its an intracellular protein and should not be in the blood. if found in the blood, that means that there has been some sort of lysis of muscles cell which is leaking troponin.
Total Troponin I = marker for total muscle breakdown
Cardiac Troponin I = marker for myocardial infarct
cross-bridge cycling
- Its a Molecular cycle of actin-myosin interaction
- Its the Mechanism of Contraction at Molecular level
- cross bridge cycling is the same thing as a molecule contraction of sarcomeres
- contraction depends on the binding of myosin heads to thin filaments (actin) at specific binding sites
- in resting-state of the sarcomere, myosin heads are blocked from binding to actin by tropomyosin, which occupies the specific binding sites (in F-actin double helical groove)
Force generation vs. sarcomere length
- on the X-axis, you have the striated spacing of a muscle.
- if you look at the spacing between the thick filaments, you have an idea of how far apart they are (how open the sarcomeres are)
- the graph at the bottom represents how long a sarcomere is. going further right (the sarcomere is wider), it’s less contracted. on the Y-axis is tension, maximal amount of force generated. As you have an increasing widening of striation, you go from almost no force being generated to a larger and larger amount of force that can be generated until you reach an optimum reach of the sarcomere where maximal force is generated.
As the sarcomere gets larger and larger, less and less force is possible. when there is maximum sarcomere length, the sarcomere si so spread out that there is no way for the cross-bridges from the myosin filament to interact with actin because the thin filaments don’t extend far enough to overlap with the myosin head.
As you shorten the sarcomere, it increases the number of cross-bridges that can interact with the actin filament.
Until you are able to get to point B and C (see image), the acting and myosin cannot interact. this is where you have a maximum level of overlap where all of the cross-bridges are able to overlap.
but when you start to get even shorter i.e C-D, there is an interference between thin filaments and myosin head because there will be incorrect directionality.
As you get closer and closer, you get more and more interference so there is less possibility for interaction until the thick filament crashes into the Z lines and no force will be generated.

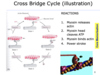
Cross Bridge Cycle (illustration)
This involves 4 stages;
- Myosin releases actin
- Myosin head cleaves ATP
- Myosin binds actin.
- Power stroke
*At the end of the cross-bridge cycle, state 4, you have the myosin head already fully pivoted so its all the way forward so it’s already dragged the actin filament along. it’s very difficult for actin and myosin to detach because ATP is required. the first time in other to reset the cycle is for the myosin head which is currently binding ADP to release ADP and form and ATP instead. once the ATP binds to the myosin head, it can then bind to the actin and likewise the myosin head is able to go back to its original position so its no longer stuck in the finished position.
*The myosin head leaves ATP into ADP + Phosphate, it goes into a high energy state. This is when its able to join to an actin filament and pulled. but the actin filament cannot be grabbed because it has troponin on its binding site, its only when there is calcium that the myosin head will release it phosphate and still hold on to ADP.
Rigor Mortis
- ATP depleted after death
- Muscle cell does not resequester Ca2+ into SR. there is an increase in Cytosolic Ca2+ because the muscle has ran out of ATP.
- Ca2+ allows cross-bridge cycle contraction continuously until the ATP and creatine phosphate run out. creatine phosphate is how the cell is replenishing its ATP. it’s like a backup gas tank.
-Without ATP, myosin stops just after the power stroke so that means that the myosin is still bound to actin because you need ATP to release actin. if you don’t have ATP, the myosin head is stuck to the actin.
- Rigor mortis ends when muscle tissue degrades after 3 days
creatine-P = creatine phosphate = a chemical used for storing energy inside the cell. ATP cannot be used for energy storage because the cellular levels of ATP must be kept at a steady level to prevent triggering biochemical reactions. Instead, extra energy in the form of ATP is stored by having ATP donate its high energy phosphate bond to creatine (to make creatine phosphate). When the level of energy in the cell is lower, then creatine phosphate can donate its high energy phosphate bond back to ADP to make ATP.
ATP, creatine phosphate and creatine phosphokinase
- Creatine is found in muscle fibres and it is the backup energy storage house.
- Creatine is phosphorylated to creatine phosphate
- This is how energy is stored in muscle
- When creating cross-bridge cycling, ATP is hydrolysed to ADP + phosphate (Pi) . creatine phosphate can donate a high energy phosphate to ADP to restore it to ATP.
- if you hold on to creatine phosphate, whenever you lose an ATP, you can regenerate it by taking the phosphate from creatine phosphate.
- ATP levels must be kept stable – buffering & regeneration. its the creatine and creatine phosphate levels that vary in the cell whereas ATP and ADP levels generally are kept in a stable range for cellular activity.
- creatine phosphate is made in the mitochondria which is making ATP hence storing much of that energy as creatine phosphate. All of this is catlysed theorugh an enzyme called enzyme creatine phosphokinase (aka CK, CPK)
Extra Note.
Creatine phosphate acts as a “energy buffer” for ATP; when a lot of ATP is formed, instead of accumulating ATP, the cell transfers the high energy phosphate bonds to creatine phosphate (ie it accumulates creatine phosphate), whilst ATP levels remain at homeostatic levels.
Creatine is a small, organic, nitrogen containing molecule
It is a store house for energy in the form of phosphate groups
The phosphates from creatine-phosphate can be transferred to ADP, making it ATP
ATP needs to be regenerated for the contraction of muscle
Energy cannot be stored as excess ATP, because ATP levels must be kept stable

Creatine vs creatinine
•Creatine is a small molecule that can accept high energy phosphate bonds from ATP
•Creatine-phosphate is the above molecule after phosphate has been added to it
•Creatine-phosphokinase (CPK) is the enzyme the adds phosphate to creatine
This is a plasma marker of muscle destruction. It is an intracellular molecule and if detected in the blood, then some cells have lysed and let its intracellular CPK into the blood.
The reason why we are able to detect it is that It is a large molecule detected by antibodies
•Creatine-kinase (CK) is just another name for creatine phosphokinase (above). They are the same thing.
•Creatinine is a diagnostic marker of kidney function. It is a breakdown product of creatine.
Calcium triggers contraction
There are 2 different calcium gradient in every cell.
- Extracellular vs cytosolic free calcium
- Between the sarcoplasmic reticulum and the free calcium
In the cytosol, the free levels of calcium are really low. outside the cell and in the SR, there is much higher levels of calcium.
Efflux of calcium from the sarcoplasmic reticulum to the cytoplasm provides most of the calcium when muscle contraction occurs. -Muscle contraction is dependent on the levels of calcium in the cytoplasm being raised and mots of the calcium comes from the SR. very little calcium come from outside the cell during muscle contraction.
Depolarisation of the cells leads to increased Ca2+
- Depolarisation is usually the cause of an increase in calcium levels
- depolarisation means the cell membrane goes from highly negative inside to positive inside
- Usually, that depolarization leads to an increase in intracellular calcium in the cytosol but that isn’t always the case.
- the rise in calcium is the ultimate cause of muscle contraction rather than depolarisation. if the cell can depolarise without raising calcium, then there will be no contraction.
How you get from depolarisation to increase in calcium
- Acetylcholine is the neurotransmitter involved. it binds at the neuromuscular junction and that leads to depolarization of the cell membrane which will lead to active nicotinic acetylcholine receptors which allows for an inward current and that is the depolarisation.
- This depolarisation is spread along the cell membrane straight towards the centre of the cell via the T tubules.
- T tubules are the invaginations that are continuous with the cell membrane its part of the cell membrane that dives deep into the cell
- this means that local action potential can trigger calcium influx from terminal Sistani
- terminal Sistani are part of the cytoplasmic reticulum which are listening out for changes in membrane potential
- the action potential goes across the membrane of the sarcoplasmic reticulum
- this then allows calcium efflux into the muscle cytoplasm.
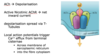
Excitation-Contraction (EC) Coupling
- Excitation is voltage changes across the membrane so its your action potential
- contraction is when the actual sarcomeres contracts
- Excitation-Contraction (EC) Coupling always means how do you get from membrane depolarization to increased levels of Calcium inside the cell cytosol
This depends on 2 main proteins
1. Ryanodine Receptor (RyR) which is in the SR membrane; it’s a calcium release channel. it allows calcium to go out of the sarcoplasmic reticulum into the cytosol. it is triggered by a voltage sensor on the calcium channel. the calcium channels are called L-type calcium channels but are also sometimes called (dihydropyridine receptor). these channels are normally sensitive to changes in voltage and down the voltage changes, these channels change conformation and because the channels are really close to the Ryanodine receptors, the channels can cause the Ryanodine receptors to open.
once you have calcium in the cytosol, you don’t want it to be there forever. that has to reserved by SERCA (smooth endoplasmic reticulum calcium ATPase) against its concentration gradient by using up ATP, so it needs ATP to run.

Tetany: molecular basis
- A muscle twitch is a single contraction of a muscle fibre.
- if you have stimulus and you stimulate the membrane of the cell
- this is time vs tension time in the X-axis and tension in the Y-axis
- for the first moment after the electrical stimulus, nothing occurs in terms of forced generation. this is called the latent period.
- It takes time for the muscle to get its act together for it to contract then after the muscles realise that there has been an electrical charge and it’s supposed to contract. then it will start to generate force and increase its contraction until it gets to a maximal level of contraction force at which point it starts to relax and generate less and less force until it generating no force at all.
- much of the contraction and drive is determined by how calcium is released. during the latent period, it takes a finite amount of time where there is almost no calcium being released. so there is no force generation.
- it takes time for that calcium to build up in the cytosol once the calcium starts to build up, contractions occur during the twitch and more and more calcium leaks out and more contraction occurs until the sarcoplasmic reticulum uses SERCA to pump calcium back into the storage. and that is what caused the relaxation
so a single action potential can cause a calcium release which leads to a twitch.
WHAT HAPPENS IF YOU HAVE FREQUENT STIMULUS?
If you have frequent action potentials, there is insufficient time for calcium resequestration to occur which leads to the summation of contraction.
eg we have this stimulus at D which causes a contraction, this contraction occurs and there is an increase in calcium and more force generation and then SERCA starts to pump back the calcium but then we stimulate the cell again and the calcium isn’t all gone yet and yet we are releasing more calcium from the SR which leads to an increase in force generation. that force generation is diminished until we continue to stimulate and stimulate. the faster and the more often yous stimulate a cell, the less chance it has to recover from the summation.
if you have an enormous number, very rapid rate of simulation, you end up with tetany. tetany is where you reach maximum force generation which means all the myosin heads are busy trying to contract, there is no time for calcium to be pumped back and it reaches the maximum and no further force can be generated. this is a very powerful contraction.

Sarcomere length
Below is a graph of sarcomere spacing (i.e. sarcomere length) vs. tension.
For each segment (e.g. between A-B or between B-C), what is the relative length of the sarcomeric A band to the sarcomeric I band?
In A-B, the I band at the length of A is so long compared to the I band. The I and is the between bit (I filament) whereas the A band is the thick filament in the centre. Keep in mind that the actual length of the A band never changes. It’s the I band that changes as sarcomeres change.
As you have less and less striation spacing, the sarcomere gets shorter and the I and gets shorter.
At the optimum levels, you probably have similar levels of A and I band .
You eventually get to an point where there is no I and and the whole sarcomere is all A band.

contractile properties of muscle cells
Muscle fibres are divided into 2 main types
1. Slow-twitch muscle fibres which are also called (type I or ‘red’ or oxidative) they have a small diam. they are slow and have a lot more ability to maintain long term contraction. the reason that they are red is because they have higher levels of myoglobin (myoglobin only have 1 subunit compared to haemoglobin with 4 subunits).
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the skeletal muscle tissue of vertebrates in general and in almost all mammals.
The reason why there are so much myoglobin is because in slow twitch fibres there are many mitochondria. mitochondria are essential for producing energy using oxygen and oxidative phosphorylation).
2. Fast-twitch muscle fibres also called (type II fibres or ‘white’).
They are nonoxidative and tend to have a wider diameter
The reason that they are wide is because they have lower levels of myoglobin
These cells tend to get more of their energy from glycolysis (breakdown of glucose into lactic acid)
its is much less energy efficient in terms of producing ATP compared to using mitochondria to go through the citric acid cycle.
*The slow and fast-twitch fibre types differ in :
1. whether they are aerobic (using O2) or anaerobic (not using O2)
2. faster calcium re-uptake (fast twitch fibres)
3. maximum tension produced (fast-twitch fibres )
4. fatigue resistance (slow twitch fibres )
basis of muscle fibre types
Type I fibres are responsible for slow and sustained actions whereas Type 2b fibres are responsible for fast movements.
There are type 2a fibres which are sort of had way between type 1 and type 2b.
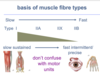
distribution of fibre types
muscles contain mixtures of fibre types and the composition depends on what the muscle action is.
In a muscle like the Salius which is primarily a postural muscle. its 80% type I which is a slow-twitch fibre and 20% type 2a. whereas something like Vastus lateralis which is used for something like running is a mixture of type I, IIA, IIX which is quite a fast type of fibre.
The proportion of muscle fibre types can depend on a person’s physical fitness. someone that is inactive is invariably gonna have more slow-twitch fibres in their muscles. Endurance athletes will have lots and lots of slow-twitch fibres whereas anaerobic fibres like sprinters will. have mostly fast-twitch fibres.

exercise and adaptation
Type I fibres are very dark in the stain image, fast-twitch are very light, the in-between is type 2a
gastrocnemius ; the chief muscle of the calf of the leg, which flexes the knee and foot. It runs to the Achilles tendon from two heads attached to the femur. you can see that most of are type 2 fast-twitch fibres.
But in a long-distance runner, it’s a different organisation. there are a lot more slow-twitch fibres.

muscle fibre types summary
You don’t have to memorise the table

co-ordination of muscle contraction
3 types of co-ordination
- Motor Units (Recruitment & the size principle)
- Tetany
- Fusion of myocytes into long myofibres
motor units
Definition of a motor unit:
It is a single alpha motor neuron and all muscle fibres it innervates.
Functions as a single contractile unit of skeletal muscle
all muscle fibres in a single motor unit are of the same type
(e.g. slow oxidative, fast oxidative, fast glycolytic).
muscle fibres which type it is gonna be depending on what it is used for by the person.
Motor Units: Variety
- motor units in large muscles is responsible for powerful gross contractions, a single lower motor neuron may synapse on 1000 fibres
- in small muscles mediating precision movement a single motor neuron may synapse with as few as 2 – 3 muscle fibres
- type and function of the lower motor neuron determines the muscle fibre,
- There are different sorts of motor units in a single muscle
Contraction: Force Generation
•Isometric force – generates a variable force while the length of the muscle remains unchanged.
“Iso” = same, “metric” = length
•isotonic force– generates a constant force while the length of the muscle changes
“tonic” = tone = tension/force iso=same
types of force generation
•Using example: picking up a drinking glass
•stage 1: isometric force– force increases, joint does not move
-Muscle Force < force of gravity –> force increases
•biceps and brachioradialis generate force by isometric contraction as muscles have not yet shortened.
•stage 2: isotonic force – force remains the same, arm moves
Glass moves upward in response to force
- an isotonic contraction starts as the force generated by the muscles overcomes gravitational and inertial forces keeping glass on the table
- glass starts to rise as the muscles shorten and the elbow bends and force generated by the muscle is constant as the glass is moving
*tips to remember, the one that ends with ENTRIC can be remembered as ELECTRIC and that is the one that can generate contraction/force without the muscle length or joints increasing.
types of muscular force generation
- Muscle contraction ≠ (necessarily) muscle shortening.
- the word muscle contraction is used for force generation but muscle contraction doesn’t mean muscle shortening.
- muscle contraction is when the muscle is generating force but if you try and generate a muscle contraction while holding the muscle in place, if you have this isometric force, you are not having muscle shortening even though you have a muscle contraction.
1. Concentric muscle force generation: this is when the muscle is becoming shorter as it contracts. i.e if you throw a ball into the air, you are contracting the bicep and that is causing the bicep to shorten and the whole arm is moving. tips to remember is to remember CONCENTRATION - when you concentrate, then you can contract a muscle and flex it.
2. Eccentric contraction (negatives): force during muscle elongation/extension. e.g. when “braking” or when the weight of the object is overwhelming – catching a ball.
•both types of force generation can occur in one behaviour
Proprioception controls force gen based on length and stretch
*if you think about a long jump. during a long jump, just at the moment that you launch yourself, you will extend the knee and that will create a lot of force as you extend the knee.
- As you extend the knew there will be an extension of the muscle and that contraction will be concentric.
- however, you will use the same muscle eccentrically when you land. if you don’t use force and holding the knew in place, it will bend. when people land, they generate a force where the knee actually extends.









