Organic Chemistry: Arenes Flashcards
What is an addition reaction?
An addition reaction occurs when two substances combine to form a single substance. For example, when hydrogen bromide is added to ethene:
HBr + C2H4 ⇔ C2H5Br
What is a substitution reaction?
A substitution reaction is whereby an atom or a group in a compound is replaced by another atom or group from another compound.
For example, when the hydroxide group in sodium hydroxide replaces the chlorine in chloroethane.
C2H5Cl + NaOH ⇔ C2H5OH + NaCl
What is an elimination reaction?
An elimination reaction is when the components of a single molecule are removed from an organic molecule and are not replaced by other atoms or groups.
For instance, a hydrogen and a halogen group are removed by an alkali, forming a C=C group. This happens with 2 bromo-propane in the presence of hot potassium hydroxide in ethanol.
CH3CHBrCH3 + KOH ⇔ CH2=CHCH3 + KBr + H2O
What is an electrophile?
An electrophile is an atom, an ion or a group that, when forming a covalent bond, attacks an electron rich site and accepts a pair of electrons from that site.
Examples include:
HBr, NO2+, CH3+ and CH3C+O
What is a nucleophile?
A nucleophile is an atom, an ion or a group that attacks a lone σ+ site, forming a covalent bond by donating a lone pair of electrons to the atom.
Examples include:
H2O, NH3, OH- and CN-.
What is a free radical?
A free radical is an atom or a group with an unpaired electron.
Examples include:
Cl• and CH3•
What are arenes sometimes known as?
Arenes are sometimes known as aromatic compounds.
What is benzene?
Benzene is a six carbon compound (C6H6) which is cyclic. The atoms are arranged in a hexagonal ring.
What is the Kekulé model?
The Kekulé shows that there is an arrangement of double and single bonds, regularly arranged.

What is wrong with the Kekulé model?
The Kekulé suggests that carbon atoms are arranged in a regular double and single bond structure.
If this was the case, benzene woudl react in certain ways, however, it doesn’t.
Double and single bonding suggests that bond lengths in benzene vary, however, bond lengths between the benzene atoms are pretty much the same length, so they can’t be double or single bonds.
Why is real benzene more stable than the Kekulé structure gives it credit for?
Real benzene is more stable because it doesnt ahve double bonds that can be attacked.
Enthalpy change calculations can be wrong when you use the Kekulé structure, so it cannot be correct.
What is the name of the other benzene structure?
The other benzene structure is known as the delocalised electron structure.
What does the delocalised electron structure look like?
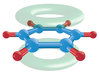
Which is more stable, benzne or cyclohexatriene?
Benzene is more stable than cyclohexatriene.
What thermochemical evidence is there for benzene being more stable than the Kekulé model?
How can the amount by which it can be stabilised be calculated?
The amount it can be stabilised can be calculated by is from the enthalpy of hydrogenation.
cyclohexene + hydrogen ⇒ cyclohexane
∆H = -119 kJ mol-1
In theory, cyclohexene should have an enthalpy change of -357kJ mol-1, so the structure cannot be as the Kekule model dictates.
benzene + hydrogen ⇒ cyclohexane
∆H = -207 kJ mol-1
What is benzene combustion reaction?
Benzene combusts in limited air to produce a smoky orange falem. Combustion is incomplete and particles of carbon form.
What addition reactions does benzene undergo?
Benzene undergoes reactions with hydrogen in the presence of a hot nickel catalyst.
Why will benzene not undergo addition reactions with halogens?
Benzene will not undergo reactions with halogens because the double bonds is not as susceptible as in alkenes.
When will benzene react bromine?
Dry benzene will react with liquid bromine in the presence of iron.
What is given off when dry benzene reacts with liquid bromine?
When dry benzene reacts with liquid bromine, steamy fumes of hydrogen bromide are given off and bromobenzene is formed (C6H5Br).
What is the mechanism for the reaction of benzene with bromine?

What happens when benzene is warmed with a mixture of sulfuric and nitric acid?
The nitro-group replaces a hydrogen atom in the benzene ring to form nitrobenzene and water.
What must react with the nitric acid to make the electrophile NO2+?
Sulfuric acid reacts with the nitric acid to form the electrophile, as well as water and a hydrogen sulphate ion (HSO4-).
H2SO4 + HNO3 ⇔ NO2+ + H2O + HSO4-
What is the reaction mechanism for the reaction of benzene and nitric acid?





