[OPTIONAL] Endocrinology Review Flashcards
Which endocrine hormones utilize the PLC mechanism? (GOAT HAG mnemonic)
GnRH + Oxytocin + ADH + TRH + Histamine + Angiotensin II + GHRH
GOAT HAG

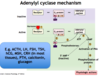
Which enzyme is responsible for the degradation of intracellular cAMP to inactive 5’ AMP in the adenylyl cyclase/cAMP pathway?
Phosphodiesterase
What are the 2 classes of homrones which are amines?
- Catecholamines (epinephrine, NE, and dopamine)
- Thyroid hormones
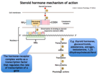
When a steroid hormone binds its receptor what occurs to the hormone-receptor complex?
Dimerizes and binds via zinc fingers to specific DNA sequences, called steroid-responsive elements (SREs)

Once the steroid hormone-receptor complex dimerizes and binds to the SREs of the target gene, what has this complex now become and what does it regulate?
A transcription factor that regulates the rate of gene transcription
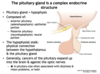
What is the epithelial and neural portion of the pituitary gland?
- Anterior pituitary (adenohypophysis) = epithelial portion
- Posterior pituitary (neurohypophysis) = neural portion
Generally cancers of the pituitary expand where and compress what?
Up into the brain and compress the optic nerves

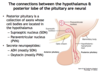
The posterior pituitary is a collection of axons whose cell bodies are located in the hypothalamus; what are these 2 cell bodies and which neuropeptides is each cell body associated with?
- Supraoptic nucleus (SON) –> mainly ADH
- Paraventricular nucleus (PVN) –> mainly oxytocin
Which 6 hormones are secreted by the anterior pituitary?
- FSH
- LH
- ACTH
- TSH
- Prolactin
- GH
*FLAT PiG*

How is the anterior pituitary connected to the hypothalamus?
Hypothalamic-hypophysial portal vessels

Which portion of the pituitary gland has both neural and endocrine connections with the hypothalamus; which has exclusively neural?
- Anterior pituitary = BOTH endocrine and neural
- Posterior pituitary = only neural
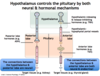
Contrast primary endocrine disorder, secondary endocrine disorder, and tertiary endocrine disorder.
- 1° disorder = due to defect in the peripheral endocrine gland
- 2° disorder = due to defect in the pituitary gland
- 3° disorder = due to defect in the hypothalamus

Gonadotrophs release what hormone(s)?
FSH and LH
Somatotrophs release what hormone?
GH
What is the most important somatomedin mediating the indirect effects of GH?
IGF-1
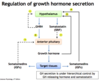
What is the effect of GH on insulin, blood glucose levels, and adipose tissues?
- Causes insulin resistance –> ↑ blood insulin
- Effects are diabetogenic –> ↑ in blood glucose levels
- ↑ lipolysis in adipose tissue

What are 2 metabolic effects of GH which are mediated by somatomedins (IGF-1)?
- ↑ protein synthesis and organ growth (↑uptake of AA’s)
- ↑ linear growth (↑ metabolism in cartilage-forming cells and chondrocytes proliferation)

What are 2 of the most potent stimuli for GH secretion?
Hypoglycemia and starvation

Conditions with excess secretion of GH are treated with what?
Somatostatin analogs –> ocreotide
What are 4 ways that GH deficiency can result?
- ↓ secretion of GHRH (hypothalamic dysf.)
- ↓ GH secretion (pituitary dysf.)
- Failure to generate somatomedins in the liver
- GH or somatomedin resistance (deficiency of receptors)
GH excess causes what if before puberty or what if after puberty?
- Before puberty = gigantism
- After puberty = acromegaly
Where does prolactin released from the anterior pituitary act and what is its function?
Acts on the hypothalamus to ↓ GnRH secretion —-> ↓ FSH and LH

What is the inhibitory and stimulatory pathway from the hypothalamus regulating prolactin release from the anterior pituitary?
- Inhibitory = Dopamine (aka PIF)
- Stimulatory = TRH
How does prolactin inhibit its own secretion?
By increasing the synthesis and secretion of dopamine from the hypothalamus (negative feedback)
What are the 2 most important stimuli for prolactin secretion?
- Breast feeding
- Pregnancy
Secretion of ADH is most sensitive to changes in what?
Plasma osmolarity
What are 6 triggers for the secretion of ADH from the posterior pituitary?
- ↑ plasma osmolarity = most important stimulus
- ↓ BP
- ↓ blood volume
- ↑ Angiotensin II
- Sympathetic stimulation
- Dehydration

In volume expansion (aka hypervolemia) what occurs with ADH secretion even in the presence of increased plasma osmolarity?
ADH secretion is inhibited
What is the major action of ADH and via what receptor?
- ↑ water permeability of principal cells in the late distal tubule and collecting duct
- Via the V2 receptor on principal cells
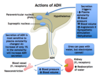
What is the effect of ADH on vascular smooth muscle and via which receptor?
Contraction of vascular smooth m. (aka vasoconstriction) via the V1 receptor

Central diabetes insipidus is caused by what; what are ADH levels like?
- Caused by: damage to posterior pituitary or destruction of hypothalamus
- LACK of ADH so will see ↓ plasma ADH
What will be seen in terms of urine production and serum osmolarity in patient with central DI?
- Large volumes of dilute urine
- Bodily fluids are concentrated i.e., ↑ serum osmolarity and ↑ serum [Na+]
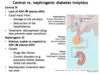
What is occuring in nephrogenic DI; what are plasma levels of ADH like?
Kidneys are unable to respond to ADH (↑ plasma ADH)
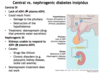
What are 2 major causes of nephrogenic DI?
- Drugs like lithium
- Chronic disorders i.e., polycystic kidney disease, sickle cell anemia

What is the Tx of Central DI vs. Nephrogenic DI?
- Central DI is treated with desmopressin (ADH analogue)
- Nephrogenic DI is treated with thiazide diuretics (desmopressin does NOT work)

What is the serum and urine osmolarity like in SIADH?
- Serum osmolarity is decreased due to excess water reabsorption by collecting ducts —> HYPOosmolarity fails to inhibit ADH release
- Urine is inappropriately concentrated (i.e., too concentrated for the serum osmolarity)

What are the 3 layers of the adrenal cortex and what are the main hormones produced by each layer?
- Zona Glomerulosa –> mineralocorticoids (aldosterone)
- Zona Fasciculata –> glucocorticoids (cortisol)
- Zona Reticularis –> androgens (DHEA)
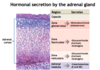
Which hormones are produced by the adrenal medulla?
Catecholamines (epinephrine and NE)

What is the precursor for all adrenal cortex hormones?
Cholesterol

Which pathway(s) of hormone synthesis in the adrenal cortex does the enzyme 17α-hydroxylase play a role in?
- Cortisol synthesis
- Androgen synthesis

Which pathway(s) of hormone synthesis in the adrenal cortex does the enzyme 21β-hydroxylase play a role in?
- Aldosterone synthesis
- Cortisol synthesis

Which pathway(s) of hormone synthesis in the adrenal cortex does the enzyme 11β-hydroxylase play a role in?
- Aldosterone synthesis
- Cortisol synthesis

For each indicate if the levels will be ↑ or ↓ in 17α-hydroxylase deficiency: mineralocorticoids, cortisol, sex hormones, BP, and [K+]
- ↑ mineralocorticoids
- ↓ cortisol
- ↓ sex hormones
- ↑ BP
- ↓ [K+]

Which enzyme deficiency is associated with ↓ androstenedione levels?
17α-hydroxylase

For each indicate if the levels will be ↑ or ↓ in 21β-hydroxylase deficiency: mineralocorticoids, cortisol, sex hormones, BP, and [K+]
- ↓ mineralocorticoids
- ↓ cortisol
- ↑ sex hormones
- ↓ BP
- ↑ [K+]

Which enzyme deficiency of the adrenal cortex is associated with ↑ renin and ↑ 17-hydroxy-progesterone?
21β-hydroxylase
For each indicate if the levels will be ↑ or ↓ in 11β-hydroxylase deficiency: mineralocorticoids, cortisol, sex hormones, BP, and [K+]
- ↓ aldosterone and ↑ 11-deoxycorticosterone (DOC) = ↑ BP
- ↓ cortisol
- ↑ sex hormones
- ↑ BP
- ↓ [K+]

Which enzyme deficiency of the adrenal cortex is associated with ↓ renin activity?
11β-hydroxylase

What will the presentation in a male vs. female child be with 17α-hydroxylase deficiency?
- Male = undescended testes; ambiguous genitalia
- Female = lack of 2° sexual development

When does 21β-hydroxylase deficiency most commonly present and how?
- In infancy w/ salt wasting or childhood (precocious puberty)
- In females = virilization

How does 11β-hydroxylase deficiency present in females?
Virilization = dev. of male physical characteristics

Which endocrine hormones utilize the AC/cAMP pathway (mnemonic from FA)
FSH + LH + ACTH + TSH + CRH + hCG + ADH + MSH + PTH

FLAT ChAMP
What is the effect of cortisol on the liver, muscle, and adipose tissue?
- Liver = gluconeogenesis
- Muscle = protein catabolism
- Adipose tissue = lipolysis

Describe the regulation of cortisol secretion (aka axis)
CRH (hypothalamus) stimulates ACTH (pituitary) –> cortisol production (adrenal fasciculata)

What is the cause of Cushing’s Syndrome; what changes are seen in CRH, ACTH and cortisol?
- Adrenal tumor
- ↓ CRH
- ↓ ACTH
- ↑ Cortisol

What is the cause of Cushing’s Disease; what changes are seen in CRH, ACTH and cortisol?
- Pituitary tumor
- ↓ CRH
- ↑ ACTH
- ↑ Cortisol
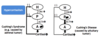
What is the cause of Addison’s Disease; what changes are seen in CRH, ACTH and cortisol?
- Caused by autoimmune disease of the adrenal gland
- ↑ CRH
- ↑ ACTH
- ↓ Cortisol
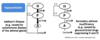
What is the cause of Secondary Adrenal Insufficiency; what changes are seen in CRH, ACTH and cortisol?
- Glucocorticoid drugs suppressing H-P axis
- ↓ CRH
- ↓ ACTH
- ↓ Cortisol

Which 2 diseases affecting cortisol secretion will have hyperpigmentation?
- Secondary (pituitary) excess
- Primary deficiency
*Processes where ACTH is elevated

What is responsible for the increased synthesis and secretion of aldosterone by stimulating cholesterol desmolase and aldosterone synthase?
Angiotensin II

How does levels of K+ affect aldosterone secretion?
- ↑ [K+] —> ↑ aldosterone
- ↓ [K+] —> ↓ aldosterone
What are the 3 main actions of aldosterone on the late distal tubule an collecting duct of the kidney?
- ↑ Na+ reabsorption
- ↑ K+ secretion
- ↑ H+ secretion
What is secreted by the α cells of the endocrine pancreas and where are these cells located?
- Secrete glucαgon
- Located near periphery of islet

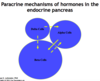
Describe the paracrine mechanism of communication between the alpha, delta, and beta cells of the pancreatic islets.
- Delta cells (somatostatin) inhibit secretion from both beta and alpha cells
- Beta cells (insulin) inhibit secretion from alpha cells
- Alpha cells (glucagon) stimulate secretion from beta cells
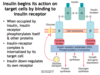
Describe the downstream effects upon glucose binding its receptor and increasing the intracellular [ATP] for the synthesis of insulin
↑ ATP –> K+ channels close —> cell depolarizes, which opens voltage-sensitivie Ca2+ channels —> ↑ intracellular Ca2+ leads to exocytosis of insulin

Explain the mechanism which makes sulfonylurea drugs (i.e., tolbutamide and glyburide) useful in the tx of T2DM
Sulfonylurea receptor (SUR) promotes the closing of ATP-dependent K+ channels –> depolarization and ↑ release of insulin

Why is C peptide useful as a diagnostic tool in the screening of endogenous B cell function?
Is secreted in equimolar amounts with insulin and excreted unchanged in the urine

Which glucose transporter on pancreatic beta cells does glucose use to enter and promote the secretion of insulin?
GLUT2

What is responsible for down-regulation of the insulin receptor by decreasing the rate of synthesis and increasing the rate of degradation?
Insulin down-regulates its own receptor
What is the effect of insulin on [K+]?
↑ K+ uptake into cells

List 10 factors which stimulate insulin secretion
- ↑ [glucose] + ↑ [AA’s] + ↑ [fatty acids] and [ketoacid]
- Glucagon
- Cortisol
- GIP
- Vagal stimulation; ACh
- K+
- Sulfonylurea drugs
- Obesity

List 6 factors which inhibit the release of insulin
- ↓ blood glucose
- Fasting
- Exercise (don’t want glucose entering cell!)
- Somatostatin
- α-adrenergic agonists
- Diazoxide

Insulin receptor signaling causes the translocation of which receptor to the cell membrane for the transport of glucose into the cell?
GLUT4
What is the incretin effect and list incretins.
- Oral glucose has a greater effect on the secretion of insulin compared to glucose given via IV
- Incretins = GLP-1 and GIP which are secreted in response to GI glucose and fat
What are 3 factors that inhibit the secretion of glucagon?
- Insulin
- Somatostatin
- ↑ fatty acid and ketoacid concentration
What is the the major factor stimulating the secretion of glucagon?
Decreased blood [glucose]
Which 2 amino acids stimulate the secretion of glucagon?
Arginine and alanine
Which hormone released from the GI tract stimulates the secretion of glucagon?
CCK
What are the 4 major actions of glucagon?
- ↑ glycogenolysis
- ↑ gluconeogenesis
- ↑ lipolysis
- ↑ ketoacid formation

What effect does ↑ phosphate concentration have on ionized Ca2+ concentration?
↑ phosphate concentration –> ↓ ionized Ca2+ concentration
Which 2 hormones stimulate an increase in bone resorption to ↑ Ca2+ concentration?
PTH and Vit D (1,25-dihydroxy)

The extracellular concentration of what ion is regulated by the same hormone that regulate Ca2+ concentration?
Phosphate (Pi)

Which hormone can cross the cell membrane in the parathyroid gland and downregulate PTH gene transcription and upregulate CaSR gene transcription?
1,25-Vitamin D

What is responsible for inhibiting the synthesis and secretion of PTH?
↑ extracellular Ca2+

What can you look for in the urine to assess the action of PTH on the kidney?
Urinary cAMP levels will be ↑ with ↑ PTH

What levels of [Ca2+], PTH and [Phosphate] stimulate 1α-hydroxylase in the renal proximal tubule to ↑ 1,25-(OH)2-cholecalciferol production?
- ↓ [Ca2+}
- ↓ [Phosphate]
- ↑ PTH

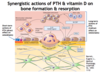
Which 2 hormones act synergistically to stimulate osteoclast activity and bone resorption?
PTH and 1,25-(OH)2-cholecalciferol
How does the short-term vs. long-term action of PTH on bone differ?
- Short-term = bone formation (via direct action on osteoblasts)
- Long-term = ↑ bone resorption (indirect action on osteoclasts mediated by cytokines released from osteoblasts)
What is the effect of vitamin D on the kidney in terms of Pi and Ca2+?
- Promotes Pi reabsorption by proximal nephrons (stimulates NPT2a expression)
- Minimal actions on Ca2+
Which type of hyperparathyroidism does this represent?

Primary Hyperparathyroidism

In terms of secondary hyperparathyroidism, blood levels of what can help you determine if the underlying cause is renal failure vs. vitamine D deficiency?
- Renal failure —> ↑ Pi
- Vitamin D deficiency —> ↓ Pi

What are the levels of PTH, Ca2+, Pi, and Vitamin D like in hypoparathyroidism?
- ↓ PTH
- ↓ Ca2+
- ↑ Pi
- ↓ Vitamin D

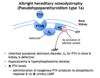
What are the levels of PTH, Ca2+, Pi, and Vitamin D like in albright hereditary osteodystrophy (pseudohypoparathyroidism type 1a)?
- ↑ PTH
- ↓ Ca2+
- ↑ Pi
- ↓ Vitamin D
What are the levels of PTH, Ca2+, Pi, and Vitamin D like in humoral hypercalcemia of malignancy?
- ↓ PTH
- ↑ Ca2+
- ↓ Pi
- ↓ Vitamin D

How does the congenital disorder pseudovitamin D-deficient rickets type I differ from type II?
- Type I = ↓ 1α-hydroxylase
- Type II = ↓ vitamin D receptor
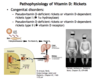
How does rickets differ from osteomalacia?
- Rickets is due to vitamin D deficiency in children; insufficient Ca2+ and phosphate are available to mineralize the growing bone.
- Osteomalacia is vitamin D deficiency in adults; new bone fails to mineralize, resulting in bendng and softening of weight-bearing bones
How does outer ring deiodination vs. inner ring deiodination of T4 in the peripheral conversion to T3 affect its activity?
- Outer ring deiodination —> active T3
- Inner ring deiodination —> inactive T3

The “Iodide-trap” for thyroid hormone biosynthesis utilizes which pump on the basolateral membrane of thryoid follicular cells?
Na+-I-cotransport (symporter)
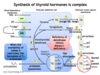
What 3 steps of thyroid hormone synthesis utilize thyroid peroxidase?
- Oxidation of I- —> I2; moves iodine through pendrin channel into the follicular lumen
- Organification of I2 w/ TG to form MIT and DIT
- Coupling reaction of MIT and DIT into T3 and T4
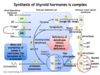
What are 2 competitive inhibitors of Na+-I- contransport, blocking the uptake of I- into follicular cells?
Thiocyanate and Perchlorate
Why is the administration of propylthiouracil (PTU) an effective treatment for hyperthyroidism?
Blocks all steps of thyroid hormone synthesis catalzyed by thyroid peroxidase

Which enzyme found inside follicular epithelial cells is responsible for deiodination of residual MIT and DIT in turn “salvaging” both I- and tyrosine for another cycle of thyroid hormone synthesis?
Thyroid deiodinase

Deficiency of which enzyme mimics dietary I- deficiency?
Thyroid deiodinase

What is the Wolff-Chaikoff effect in regards to thyroid hormone synthesis?
High levels of I- inhibit organification and synthesis of thyroid hormones

Thyroxine-binding protein (TBG) has a higher affinity for what type of thyroid hormone?
T4

In hepatic failure there is decreased production of thyroxine-binding protein (TBG) and what effect does this have on levels of free T3 and T4 and the synthesis of thyroid hormones?
- Causes an ↑ in the level of free T3 and T4 because less is bound to TBG
- This ↑ in free thyroid will negatively feedback to thyroid to inhibit synthesis of thyroid hormones

What is the effect of pregnancy of levels of TBG, free T3,T4 and the synthesis of thyroid hormone?
- ↑ levels TBG —> ↑ bound T3, T4 —> ↓ free T3, T4
- Transient ↓ T3, T4 causes ↑ in the synthesis and secretion of T3, T4 by the thyroid
- Overall = ↑ total T3, T4, but levels of free, physiologically active, thyroid hormone are normal = clinically euthyroid

Release of TSH from the anteror pituitary is positively and negatively regulated by what?
- (+) regulated by TRH from the hypothalamus
- (-) regulated by free T3

What is the effect of hyper- and hypothyroidism on bone?
- Hyperthyroidism = osteoporosis
- Hypothyroidism = stunted growth

Explain how thyroid-stimulating immunoglobulins which underlie the pathophysiology of Graves Disease affect the HPT axis and effect levels of TSH and thyroid hormones?
- TSI’s stimulate the TSH receptor without TSH hormone
- Leads to an ↑ in circulating thyroid hormones which inhibit the secretion of TSH



