Neuroimaging Flashcards
Ventricular Anatomy
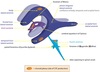
Cerebral Edema
Edema within the brain can be caused by cell death, altered capillary permeability, or hemodynamic forces.
Cytotoxic Edema
Cytotoxic edema is cell swelling caused by damaged molecular sodium-potassium ATPase ion pumps. It can affect both gray and white matter.
Cytotoxic edema is caused by cell death, mot commonly due to infarct. Water ions trapped inside swollen cells feature reduced diffusivity.
Vasogenic Edema
Vasogenic edema is interstitial edema caused by increased capillary permeability. It is seen primarily in the white matter, as there is more interstitial space.
Vasogenic edema is caused most commonly by neoplasm, infection, or infarct.
Interstitial Edema
Interstitial edema is caused by imbalances in CSF flow, most commonly due to obstructive hydrocephalus.
Interstitial edema presents on imaging as periventricular fluid, often called “transependymal flow of CSF”, even though it is unlikely that the CSF actually flows across the ependymal cells lining the ventricles.
Herniation
The total volume in the skull is fixed. Increases in intracranial pressure may lead to herniation across a dural fold.
Herniation may be due to a mass lesion (such as a neoplasm or hematoma) or may be due to edema secondary to a large stroke. Because the volume of the posterior fossa is especially limited, cerebellar infarcts are prone to herniation.
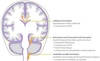
Cerebellar tonsillar herniation
Downward displacement of the cerebellar tonsils through foramen magnum causes compression of the medulla.
Compression of medullary respiratory centers is often fatal.
Basal cisterns
The basal cisterns, also known as the perimesencephalic cisterns, are CSF–filled spaces surrouding the midbrain and pons.
Compression or effacement of the basal cisterns may be a sign of the impending or actual herniation.
Blood Brain Barrier (BBB) and enhancement
Micro or macro disruption of the blood brain barrier (BBB) produces parenchymal enhancement after contrast administration, which may be secondary to infection, inflammation, neoplasm, trauma, and vascular etiologies.
The BBB is formed by astrocytic foot processes of brain capillary endothelial cells and prevents direct communication between the systemic capillaries and the protected extracellular fluid of the brain.
Several CNS regions do not have a blood brain barrier, and therefore normally enhance: Choroid plexus, Pituitary and pineal glands, Tuber cinereum (controls circadian rhythm, located in the inferior hypothalamus). Area postrema (controls vomiting, located at inferior aspect of 4th ventricle).
The dura also lacks a blood brain barrier, but does not normally enhance. This phenomenon is subsequently explened in the section on pachymeningeal (dural) enhancement.
Vascular enhancement is due to a localized increase in blood flow, which may be secondary to vasodilation, hyperemia, neovascularity, or arteriovenous shunting. On CT, the arterial phase of contrast injection (for instance a CT angiogram) mostly shows intravascular enhancement. Parenchymal enhancement, including the dural folds of the falx and tentorium, is best seen several minutes after the initial contrast bolus.
On MRI, routine contrast-enhanced sequences are obtained in the parenchymal phase, several minutes after injection. Most intracranial vascular MRI imaging is performed with a noncontrast time of flight technique.
Intracranial enhancementmay be intra- or extra-axial. Extra-axial structures that may enhance in pathologic conditions include the dura (pachymeninges) and arachnoid (leptomeninges).
Perfusion
Perfusion MR is an advanced technique where the brain is imaged repeatedly as a bolus of gadolinium contrast is injected. The principle of perfusion MR is based on the theory that gadolinium causes a magnetic field disturbance, which (counterintuitively) transiently decreases the image intensity. Perfusion images are echo-planar T2* images, which can be acquired very quickly.
Perfusion MR may be used for evaluation of stroke and tumors.
Periventricular enhancement (intra-axial)
Enhancement of the subependymal surface can be either neoplastic, infectious, or demyelinating in etiology.
Primary CNS lymphoma is a malignant B-cell neoplasm that can have diverse presentations including periventricular enhancment, solitary brain mass, or multiple brain masses.
Primary CNS lymphoma is hyperattenuating on CT and demonstrates low ADC and low signal intensity on T2-weighted MRI due to hypercellularity.
Primary CNS lymphoma rarely involves the meninges. In contrast, the meninges (both pachymeninges and leptomeninges) are commonly involved when systemic lymphoma spreads to the brain.
CNS lymphoma tends to be centrally necrotic in immunocompromised patients, but usually enhances homogeneously in immunocompetent patients.
Infectious ependymitis is most commonly caused by cytomegalovirus. Infectious ependymitis usually features thin linear enhancment along the margins of the ventricles.
Primary glial tumor may cause periventricular enhancement.
Multiple sclerosis may affect the subependymal surface. Although the majority of demyelinating lesions do not enhance, an active plaque may demonstrate enhancement.
Gyriform enhancement (intra-axial)
Superficial enhancement of the cortical (gyral) surface of the brain can be due to either cerebral infection, inflammation, or ischemia.
Herpes encephalitis is a serious necrotizing infection of the brain parenchyma due to reactivation of latent HSV-1 infection within the trigeminal ganglion. The medial temporal lobes and cingulate gyrus are usually affected first and demonstrate gyral enhancement due to inflammation, petechial hemorrhage, and resultant BBB breakdown. The involved areas typically also demonstrate reduced diffusivity.
Meningitis may cause gyral enhancment in addition to the more typical leptomenigneal enhancement (subsequently discussed).
Subacute infarct can demonstrate gyriform enhancement lasting approximately 6 days to 6 weeks after the initial ischemic event.
In contrast to the gyriform enhancement of subacute infarct, an acute infarct may demnonstrate vascular enhancement due to reactive collateral vasodilation and resultant hyperemia.
Posterior reversible encephalopathy syndrome (PRES) is a syndrome of vasogenic white matter edema triggered by altered autoregulation that may demonstrate gyral enhancment. PRES may rarely exhibit restricted diffusion.
Nodular subcortical enhancment (intra-axial)
Nodular intra-axial enhancement is most commonly due to metastatic disease.
Hematogenously disseminated metastatic disease is commonly found at the subcortical gray-white junctions. Tumor emboli become “stuck” at the junction between the simple vasculature of the white matter and the highly branching vasculature of the gray matter.
Edema is almost always present with metastatic disease of the gray-white junction, although slightly more distal cortical metastases (e.g. pelvic malignancy spread via the Batson preverterbral venous plexus) leads to posterior fossa disease by transit through the retroclival venous plexus.
Ring enhancement (intra-axial)
Peripheral (ring) enhancment is a common presentation with a broad range of differential diagnoses. The two most common causes are high-grade neoplasm and cerebral abscess.
The mnemonic MAGIC DR (metastasis, abscess, glioma, infarct, contusion, demyelination, and radiation) may be helpful to remember the wide range of etiologies for ring enhancement, although it is usually possible to narrow the differential basedon the pattern of ring enhancement combined with additional MRI sequences and clinical history.
Metastasis: Hematogenous metastases are typically found at the subcortical gray-white junction. Metastases are often multiple, but smaller lesions may not be ring-enhancing.
Abscess: A pyogenic abscess is formed as a result of organization and sequestration of an infection, featuring a central region of viscous necrosis.
The key imaging findings of abscess are reduced diffusivity (bright on DWI and dark on ADC) caused by high viscosity of central necrosis and a characteristic smooth, hypointense rim on T2-weighted images.
Glioma: High grade tumors such as glioblastoma typically have a thick and irregular wall.
Multivoxel MRI spectroscopy will be abnormal outside the margin of an enhancing high grade glial neoplasm secondary to nonenhancing infiltrative tumor. This is in contrast to a demyelinating lesion, abscess, and metastasis, where the spectral pattern returns to normal at the margin of the lesion.
Perfusion MRI demonstrates elevated perfusion in a high grade glioma.
Infarct: Although subacute cortical infarcts often demonstrate gyral enhancment, ring enhancement can be seen in subacute basal ganglia infarcts.
In contrast to neoplasm and infection, a subacute infarct does not have significant mass effect.
Contusion: Both traumatic and nontraumatic intraparenchymal hemorrhage can show ring enhancement in the subacute to chronic stage.
Demyelinating disease: The key finding in ring-enhancing demyelinating disease is lack of significant mass effect. The “ring” of enhancement is often incomplete and “C” shaped.
Multiple sclerosis is the most common demyelinating disease. Enhancement suggests active disease.
Although the typical finding is an incomplete rim of enhancement, tumefactive demyelinating disease can look identical to a high-grade tumor.
Radiation necrosis may look identical to a high-grade tumor. On perfusion, cerebral blood volume is generally low in radiation necrosis and typically increased in a high grade glioma.
Pachymeningeal (dural) enhancement (extra-axial)
The pachymeninges (pachy means thick - a “thick-skinned” elephant is a pachyderm) refers to the dura mater, the thick and leather-like outermost covering of the brain.
In addition to surrounding the surface of the brain, the dura forms several reflections, including the falx, tentorium, and cavernous sinus.
The dura does not have a blood brain barrier. Although contrast molecules normally diffuse into the dura on enhanced CT or MRI, dural enhancement is never visualized on CT and is only viscualized on MRI in pathologic situations.
Dural enhancement is not seen on CT because both the skull and adjacent enhancing dura appear white.
Enhancement of normal dura is not visible on MRI because MRI visualization of enhancement requires both water protons and gadolinium. Although gadolinium is present in the dura, there are normally very few water protons. However, dural pathology often causes dural edema, which provides enough water protons to make the gadolinium visible. Therefore, dural enhancement on MRI is an indication of dural edema rather than BBB breakdown.
Differential diagnosis of pachymeningeal enhancement
Intracranial hypotension: Prolonged decrease in cerebrospinal fluid pressure can lead to vasogenic edema in the dura.
Intracranial hypotension clinically presents as a postural headache exacerbated by standing upright.
Intracranial hypotension may be idiopathic or secondary to CSF leak from surgery or lumbar puncture.
Imaging shows thick, linear dural enhancement, enlargement of the pituitary gland, and “sagging” of the cerebellar tonsils. There may also be subdural hemorrhage due to traction effect on the cerebral veins.
Postoperative: Dural enhancment may be seen postoperatively.
Post lumbar puncture: Diffuse dural enhancement is occasionally seen (<5% of the time) after routine lumbar puncture.
Meningeal neoplasm, such as meningioma, can produce a focal area of dural enhancement called a dural tail, due to reactive changes in the dura. Metastatic disease to the dura, most commonly breast cancer in a female and prostate cancer ina male, can cause irregular dural enhancement.
Granulomatous disease, including sarcoidosis, tuberculosis, and fungal disease, can produce dural enhancement, typically of the basal meninges (meninges of the skull base).
Leptomeningeal (pia-arachnoid) enhancement (extra-axial)
The leptomeninges (lepto means thin or narrow) include the pia and arachnoid.
Leptomeningeal enhancment follows the undulating contours of the sulci as it includes enhancement of both the subarachnoid space and the pial surface of the brain.
The differential diagnosis of FLAIR hyperintensity in the subarachnoid space overlaps with the differential for leptomeningeal enhancement. Subarachnoid FLAIR hyperintensity may be due to:
Meningitis and leptomeningeal carcinomatosis both have increased subarachnoid FLAIR signal and leptomeningeal enhancement
Subarachnoid hemorrhage manifests as increased subarachnoid FLAIR signal, without leptomeningeal enhancement. Blooming artifact on GRE and SWI from blood products will help differentiate subarachnoid hemorrhage from carcinomatosis.
Subarachnoid FLAIR signal is artifactually increased when the aptient is on oxygen or propofol therapy, without abnormal enhancement.
Differential diagnosis of leptomeningeal enhancment
Meningitis (either bacterial, viral, or fungal) is primary consideration when leptomeningeal enhancment is seen.
Leptomeningeal enhancment in meningitis is caused by BBB breakdown due to inflammation or infection.
Fine, linear enhancement suggests bacterial or viral meningitis.
Thicker, nodular enhancment suggests fungal meningitis.
Leptomeningeal carcinomatosis, also called carcinomatous meningitis, is spread of neoplasm into the subarachnoid space, which may be due to primary brain tumor or metastatic disease.
CNS neoplasms known to cause leptomeningeal carcinomatosis include medulloblastoma, oligodendroglioma, chroid plexus tumor, lymphoma, ependymoma, glioblastoma, and germinoma. Mnemonic MOCLEGG or GEMCLOG
Metastatic tumros known to cause carcinomatosis include lymphoma and breast cancer.
Viral encephalitis may produce cranial nerve enhancement within the subarachnoid space.
Slow vascular flow may mimic leptomeningeal enahcnement at first glance, but a careful examination shows the distinction. Slow flow appears as an intravascular distribution of FLAIR hyperintesity due to “unmasking” of the inherent high signal of blood, which remains int eh plane of imaging as the entire pulse sequence is obtained.
Slow flow of peripheral vessels in moyamoya disease causes the ivy sign.
Tumor-related complications
The three emergent complications of a brain tumor are the three H’s: Hemorrhage, Hydrocephalus, and Herniation. CT is a good screening method to evaluate for these complications.
Hemorrhage: Primary or metastatic brain tumors are often associated with neovascularity and tumoral vessels are more prone to hemorrhage than normal vasculature.
The most common primary brain tumor to hemorrhage is a glioblastoma.
Hemorrhagic metastases include melanoma, renal cell carcinoma, thyroid carcinoma, and choriocarcinoma. Although breast and lung cancer metastases are less frequently hemorrhagic on a case-by-case basis, these two malignancies are so common that they should also be considered in the differential of a hemorrhagic metastasis.
Mass intra- or extra-axial
After evaluation for emergent complications, the next step is to determine if the lesion in intra- or extra-axial. This distinction can sometimes be quite tricky.
Although metastases may be either intra- or extra-axial, the differential diagnosis for each space is otherwise completely different.
Findings of an extra-axial mass include a CSF cleft between the mass and the brain, buckling of gray matter, and gray matter interposed between the mass and white matter.
Findings of an intra-axial mass include absence of intervening gray matter between the mass and the white matter.
The presence of white matter edema is not specific to intra-axial masses. In particular, meningioma (an extra-axial dural neoplasm) is known to cause white matter edema of underlying brain.
Meningeal enhancement is seen more commonly in extra-axial masses (most commonly meningioma), but can also be seen in intra-axial masses.
Tumors hypointense on T2-weighted images include
Metastases containing desiccated mucin, such as some gastrointestinal adenocarcinomas. Not that mucinous metastases to the brain can have variable signal intensities on T2-weighted images, depending on the water content of the mucin. Hydrated mucin is hyperintense on T2-weighted images.
Hypercellular tumors, including lymphoma, medulloblastoma, germinoma, and some glioblastomas.
Tumors hyperintense on T1-weighted images include
Metastatic melanoma (melanin is hyperintense on T1-weighted images).
Fat-containing tumors, such as dermoid or teratoma.
Hemorrhagic metastasis (including renal cell, thyroid, choriocarcinoma, and melanoma).
Some tumors contain cystic compoments which are isointense to CSF on all sequences. (Note that cysts tend to be at the peripherey of enhancing low grade tumors. In contrast, although intra-tumoral necrosis of a high-grade tumor may also follow CSF signal, necrosis tends to be surrounded by enhancing tumor.
Glial Cells
A glioma is a primary CNS tumor that arises from a glial cell. Glial cells include astrocytes, oligodendrocytes, ependymal cells, and choroid plexus cells.
Glioma is not a synonym for a “brain tumor”. Only a tumor that arises from one of the aforementioned glial cells can accurately be called a glioma.
The normal functions of an astrocyte are to provide biochemical support to the endothelial cells that maintain the blood brain barrier, to maintain extracellular ion balance, and to aid in repair after a neurona injury.
Astrocytes are normally located throughout the entire brain (primarily in the white matter) and spinal cord.
The normal funnction of an oligodendrocyte is to maintain myelin around CNS axons. A single oligodendrocyte can maintain the myelin of dozens of axons. The counterpart in the peripheral nervous system is the Schwann cell, which maintains myelin around a single peripheral nerve. Unlike the oligodendrocyte, each Schwann cell is in charge of only a single axon.
Oligodendrocytes are normally located throughout the entire brain and spinal cord.
The normal function of an ependymal cell is to circulate CSF with its multiple cilia.
Ependydmal cells line the ventricles and central canal of the spinal cord.
The normal function of a choroid plexus cells is to produce CSF. A choroid plexus cell is a modified ependymal cell.
Choroid plexus cells are located intraventricularly, in the body and temporal horn of each lateral ventricle, roof of the third ventricle and roof of the fourth ventricle.
Grade I Astrocytoma
Juvenile pilocytic astrocytoma (JPA)
Juvenile pilocytic (hair-like) astroctyoma (JPA) is a benign World Health Organization (WHO) grade I tumor seen typically in the posterior fossa in children.
Imaging shows a well-circumscribed cystic mass with an enhancing nodule and relatively little edema. When in the posterior fossa, JPA may compress the fourth ventricle.
JPA can also occur along the optic pathway, with up to 1/3 of optic pathway JPA associated with neurofibromatosis type 1. Posterior fossa JPA is not associated with NF1.
Fibrillary Astrocytoma
Fibrillary astrocytomas are infiltrative tumors that include low-grade astrocytoma, anaplastic astrocytoma, and glioblastoma multiforme (GBM).
Astrocytomas can occur in the brain or the spinal cord.
Low-grade astrocytoma is a WHO grade II tumor that typically presents as a hyperintense mass on T2-weighted images, without enhancement. Imaging findings may be subtle.
Anaplastic astrocytoma is a WHO grade III tumor. It features a range of appearances from thickened cortex (similar to low-grade astroctyoma) to an irregularly enhancing mass that may appear identical to glioblastoma. The natural history of the disease is eventual progression to glioblastoma.
Glioblastoma multiforme (GBM) is an aggressive WHO grade IV tumor of older adults. It is the most common primary CNS malignancy. GBM has a highly variable appearance (“multiforme”) but typically presents as a white matter mass with heterogenous enhancement and surrounding nonenhancing T2 prolongation. Most of the surrounding T2 prolongation is thought to represent infiltrative tumor.
GBM is an infiltrative disease that spreads through white matter tracts, through the CSF, and subependymally. Subependymal spread describes spread within the walls of the ventricles under the ependymal cells.
A GBM that crosses the midline via the corpus callosum is called a butterfly glioma. The differential diagnosis of a transcallosal mass includes glioblastoma, lymphoma, and demyelinating disease.
Other Gliomas
Gliomatosis cerebri is a diffuse infiltrative mid-grade (WHO II or III) astroctyoma that affects multiple lobes.
Diagnostic criteria include involvement of at least two lobes plus extra-cortical involvement of structures such as the basal ganglia, corpus callosum, brainstem, or cerebellum.
Gliomatosis has a poor prognosis and may degenerate into GBM.
The typical imaging appearance is diffuse T2 prolongation throughout the involved brain. Diffuse T2 prolongation can be seen in several entities, typically in immunocompromised patients, including lymphoma, progressive multifocal luekoencephalopathy (demyelination caused by JC virus), and AIDS encephalopathy.
Gliomatosis exerts mass effect but typically does not enhance.
Oligodendroglioma is a WHO grade II tumor that usually presents as a slow-growing cortical-based mass.
The typical patient is a young to middle-aged patient presenting with seizures.
Oligodendrogliomas have a propensity to calcify (approximately 75% calcify). Variants such as oligoastroctyoma and anaplastic oligodendroglioma are much more aggressive. Oligoastroctyoma is a mixed tumor with an astrocytic component. Although oligoastrocytoma can degenerate into GBM, typically prognosis is better than a pure GBM. Anaplastic oligodendroglioma is indistinguishable from GBM on imaging and has a poor prognosis.
Ependymoma is a tumor of ependymal cells that tends to occur in the posterior fossain children and in the spinal cord in older adults.
The pediatric posterior fossa ependymoma has been called the toothpaste tumor for its propensity to fill the fourth ventricle and squeeze through the foramina of Magendie or Luschka into the adjacent basal cisterns. Medulloblastoma, the most common pediatric brain tumor, also usually arises in the posterior fossa but does not typically squeeze through the foramina.
The adult spinal ependymoma can occur anywhere in the intramedullary spinal cord. The main differential diagnosis of an intramedullary spinal cord mass is an astrocytoma, which tends to occur in younger patients. It is not possible to reliably differentiate spinal cord ependymoma from astrocytoma on imaging.
Non-Glioma Primary Brin Tumors
Lhermitte-Duclos, also called dysplastic cerebellar gangliocytoma, is a WHO grade I cerebellar lesion that is part hamartoma and part neoplasm.
Lhermitte-Duclos is almost always seen in association with Cowden syndrome (multiple hamartomas and increased risk of several cancers).
The classical imaging finding is a corduroy or tiger-striped striated lesion in the cerebellar hemisphere. Enhancement is rare.
Embryonal Tumors
Embryonal Tumors represent a spectrum of WHO grade IV, aggressive childhood malignancies that are known as primitive neuroectodermal tumors (PNET). Intracranial PNET tumors are more commonly located in the posterior fossa but may occur supratentorially.
Atypical teratoid/rhabdoid tumor (ATRT) is a WHO IV, aggressive tumor that may appear similar to medulloblastoma, but occurs in slightly younger patients. The majority occur in the posterior fossa. ATRT is associated with malignant rhabdoid tumor of the kidney.
Medulloblastoma is a WHO grade IV tumor of small-blue-cell origin. It is one of the most common pediatric brain tumors.
Medulloblastoma most commonly occurs in the midline in the cerebellar vermis. It is slightly hyperattenuating on CT due to its densely packed cells and is accordingly hypointense on T2-weighted images and has low ADC values. The tumor is avidly enhancing and may appear heterogenous due to internal hemorrhage and calcification. The low ADC values can be a useful finding to differentiate medulloblastoma from ependymoma and pilocytic astroctyoma, the two other most common childhood posterior fossa tumors.
Leptomeningeal metastaic disease is present in up to 33% of patients. Sugar-coating (Zuckerguss) is icing-like enhancement over the brain surface. Imaging of the entire brain and spine should be performed prior to surgery.
When medulloblastoma occurs in a young adult (as opposed to a child), the tumor tends to arise eccentrically in the posterior fossa, from the cerebellar hemisphere.
Tumors with a cyst and an enhancing nodule
A few low-grade, fluid-secreting tumors present as a cyst with an enhancing mural nodule. (Juvenile pilocytic astrocytoma, Hemangioblastoma, Pleomorphic Xanthroastrocytoma (PXA), Ganglioglioma)
Hemangioblastoma is a highly vascular WHO grade I tumor associated with von Hippel-Lindau (VHL) syndrome that occurs most commonly in the cerebellum, medulla, or spinal cord. It only rarely occurs supratentorially.
Although associated with VHL, only 30% of patients with hemangioblastoma have VHL. Hemangioblastoma in a patient with VHL has a worse prognosis.
The classic appearance of hemagioblastoma is a cystic mass with an enhancing mural nodule. Prominent vessels are often seen as tubular areas of flow void. Less commonly, a hemangioblastoma may be solid or hemorrhagic.
When in the spinal cord, hemangioblastoma is often associated with a syrinx.
Pleomorphic Xanthroastroctyoma (PXA) is a low-grade WHO grade II astroctyoma variant.
PXA is a rare tumor of childhood and adolescents, typically with history of chronic epilepsy.
The most common location of PXA is the temporal lobe, where it typically presents as a supratentorial cortical cystic mass with an enhancing mural nodule. The overlying dura may be thickened and enhancing.
The main differential consideration, both by imaging and clinical presentation, is ganglioglioma; however, ganglioglioma does not usually cause dural thickening.
Ganglioglioma is a rare slow-growing neuroglial tumor that typically presents in an adolescent or young adult with medically refractory temporal lobe epilepsy.
Ganglioglioma characteristically appears as a temporal lobe cyst and enhancing mural nodule, often with calcification. Ganglioglioma may cause calvarial remodeling and scalloping.
Intraventricular Tumors
Central neurocytoma is a low-grade tumor likely of neuronal origin that occurs in young adults, from teenagers to young middle-aged patients. Prognosis is excellent.
Typical imaging appearance is a lobulated mass attached to the septum pellucidum, with numerous intratumoral cystic areas. Calcification is common.
Choroid plexus papilloma is rare intraventricular tumor. Choroid plexus papilloma is a low-grade (WHO I) neoplasm arising from choroid plexus epithelial cells. It is the most common brain tumor in babies < 1 year old, but may also occur in adults.
T2-weighted images show a lobulated, heterogenous or hyperintense mass that avidly enhances on T1-weighted MRI.
In children, the atrium of the lateral ventricle is the most common location.
In adults, the fourth ventricle is the most common location.
Less commonly, choroid plexus papilloma may arise from the third ventricle or cerebromedullary angle.
Choroid plexus papilloma and carcinoma (WHO grade III) are not reliably distinguishable.
Intraventricular meningioma appears as a solid mass, typically in the trigone of the lateral ventricle. It tends to ccur in older patients, similar to other meningiomas.
Intraventricular meniniomas are typically hypercellular and homogenously enhance, distinguishing them from other intraventricular neoplasms.
Subependymal giant cell astrocytoma (SEGA) is a low-grade (WHO I) astroctyoma variant that is associated with tuberous sclerosis. Other findings in tuberous sclerosis include subependymal nodules and hamartomas (cortical and subcortical). SEGA classically is an enhancing mass in the lateral ventricle near the foramen of Monro.
Subependymoma is a nonenhancing low-grade tumor of unclear origin thought to arise from subependymal astrocytes, ependymal cells lining the ventricles, or common precursor cells.
Subependymoma is a tumor of middle-aged and older adults. It is often found incidentally.
The most common locations are the obex of the 4th ventricle (inferior 4th ventricle) or at the foramen of Monro in the lateral ventricle. The tumor usually doesn’t enhance. Despite their similar names, subependymoma is not related to subependymal giant cell astroctyoma (discussed above, associated with tuberous sclerosis) or with ependymoma.
Primary CNS Lymphoma: Overview
Primary CNS lymphoma is lymphoma isolated to the CNS, most commonly diffuse large B-cell lymphoma. Immature blast cells form lymphoid aggregates around small cerebral blood vessels in a periventricular location. Note that the brain does not contain native lymphoid tissue.
PCNSL is known to “melt away” with chemoradiation but tends to recur aggressively.
The appearance of PCNSL depends on the immune status of the patient. Regardless of immune status, however, key imaging findings are a periventricular location and high cellularity (hyperattenuating on CT, relatively hypointense on T2-weighted images, and reduced diffusivity).
Primary CNS lymphoma: Immunocompetent patient
In an immunocompetent patient, PCNSL typically presents as an enhancing periventricular mass, often crossing the corpus callosum to involve both hemispheres. Involvement of the frontal lobes and basal ganglia is most common.
The differential diagnosis for a mass involving the corpus callosum includes lymphoma, glioblastoma multiforme, and demyelinating lesion.
PCNSL in an immunocompetent individual usually enhances homogenously, without central necrosis. This is in contrast to PCNSL in an immunocompromised patient, where central necrosis is typical.
Primary CNS lymphoma: Immunocompromised patient
In an immunocompromised patient, PCNSL typically presents as a periventricular ring-enhancing lesion in the basasl ganlgia. The ring enhancement is caused by central necrosis. The two primary differential considerations for a ring-enhancing basal ganglial mass in an immunocompromised patient are lymphoma and toxoplasmosis.
Several clinical and imaging options are available to differentiate between lymphoma and toxoplasmosis: Empirical anti-toxoplasmosis therapy and short-interval follow-up. Thallium scanning: CNS lymphoma is thallium avid and toxoplasmosis does not take up thallium. PET: CNS lymphoma tends to be high-grade and metabolically active. Toxoplasmosis usually does not have avid FDG uptake. Perfusion scanning: CNS lymphoma has increased relative cerebral blood volume while toxoplasmosis is hypovascular. Note that lymphoma and toxoplasmosis cannot be reliably differentiated by enhancement. Intra-axial enhancement is a measure of capillary leakage, not perfusion. Both will enhance.
Secondary CNS lymphoma
Secondary CNS lymphoma represents involvement of the CNS in a patient with known extra-cerebral lymphoma. Secondary CNS lymphoma tends to involve the meninges and may cause leptomeningeal carcinomatosis or epidural cord compression.
Less commonly, secondary CNS involvement of lymphoma may present as a parenchymal mass.
Metastatic disease to the brain
The most common primary tumors to cause parenchymal metastasis are lung, breast, and melanoma.
Most metastasis are hematogenous and arise at the gray-white junction, where there is a caliber change in the small arterioles.
Enhancement is universal, as capillaries produced by an extra-CNS primary tumor do not have a functioning blood brain barrier.
Larger metastases often feature marked edema, while small metastases may present as tiny enhancing foci apparent only on the post-contrast images.
Dural Neoplasms
Meningioma is by far the most common extra-axial tumor. It arises from meningoepithelial cells called arachnoid “cap” cells. Meningiomas typically occur in elderly adults with a female predominance and are most often asymptomatic.
The vast majority are benign, but 1-2% are anaplastic or malignant. Both benign and malignant meningiomas can metastasize, although this is uncommon.
Multiple meningiomas are seen in neurofibromatosis type 2 or following radiation therapy.
Meningiomas can occur anywhere in the neuraxis, but are most commoly supratentorial and parasagittal.
Meningiomas may also be intraventricular (in the trigone/atrium of the lateral ventricle) or intra-osseous. Intra-osseous meningioma may mimic fibrous dysplasia.
On noncontrast CT, meningiomas are usually hyperattunating relative to brain and approximately 25% calcificy.
On MRI, appearance can be variable with iso- or slightly hypointense signal on T1-weighted images and variable signal intensity on T2-weighted images. There is typically a broad-based attachment to the dura.
Meningiomas avidly enhance. An enhancing dural tail is thought to be due to vasoactive substances released by the meningioma rather than tumor spread to the dura.
Despite the extra-axial location of most meningiomas, there may be extensive white matter edema, thought to be due to vasoactive factors and a pial vascular supply. There is often a discordance between the size of meningioma and degree of white matter edema, with severe edema possible even with a very small tumor.
The most common tumors to metastasize to the dura are breast (most common), lymphoma, small cell lung cancer, and melanoma.
Posterior Fossa Masses: Differential Diagnosis

Overview and anatomy of the CPA
The cerebellopontine angle (CPA) is region between the pons and cerebellum and the posterior aspect of the petrous temporal bone. Important structures of the CPA include the 5th (trigeminal), 7th (facial), and 8th (vestibulocochlear) cranial nerves, and the anterior inferior cerebellar artery (AICA).
Most lesions of the CPA are extra-axial and lcoated in the CPA cistern itself, although some may arise in the internal adutory canal (IAC), temporal bone, or rarely intra-axially from teh pons or cerebellum. CPA masses are more common in adults.

Schwannoma
Schwannoma of the vestibulocochlear nerve, also known as a vestibular schwannoma, is by far the most common cerebellopontine angle mass, representing greater than 75% of all CPA masses.
Vestibular schwannoma is hyperintense on T2-weighted images and avidly enhances. The characteristic ice cream cone appearance describes the “cone” protruding through (and widening) the porous acoustics and the “ice cream” exerting mass effect on the cerebellar-pontine junction. Schwannoma may become cystic, especially when larger.
Schwannomas of other cranial nerves in the CPA, including the facial or trigeminal nerves, are less common. Trigeminal schwannoma may extend into Meckel’s cave.
Meningioma
Although meningioma is overall the most common extra-axial mass in adults, it is only the second most common mass of the CPA, representing approximately 10-15% of all CPA masses.
Meningiomas often feature a short segment of dural enhancement and may induce adjacent bony hyperostosis. Approximately 20% calcify, in contrast to schwannomas where calcification is rare.
In contrast to schwannoma, a CPA meningioma does not enlarge the porous acousticus.
Arachnoid cyst
An arachnoid cyst is a benign CSF-filled lesion that is usually congenital. Although most arachnoid cysts are supratentorial, the cerebellopontine angle is the most common infratentorial location.
An arachnoid cyst will follow CSF signal on all sequences, including complete suppression on FLAIR. Unlike an epidermoid cyst, an arachnoid cyst does not have restricted diffusion.
Aneurysm
Vertebrobasilar aneurysm (arising from the posterior inferior cerebellar artery, anterior inferior cerebellar artery, vertebral artery, or basilar artery) may appear as a well-defined, avidly enhancing CPA lesion and may be initially mistaken for a schwannoma or meningioma on contrast-enhanced CT.
On MRI, clues to a vascular etiology would be flow void and pulsation artifacts. MRA or CTA are diagnostic.
Epidermoid cyst
An epidermoid cyst is a congenital lesion arising from ectopic ectodermal epithelial tissue.
Epidermoids progressively enlarge from desquamation of keratinized epithelium lining the cyst. The mass characteristically insinuates in between structures, encasing cranial nerves and vessels. Gross pathology featrures a characteristic “cauliflower-like” surface.
On CT, epidermoid cyst may mimic arachnoid cyst and appear as a water-attenuation cystic structure. On MRI, an epidermoidcyst has similar signal characteristics to CSF on T1- and T2-weighted images. Unlike arachnoid cyst, an epidermoid does not usually suppress on FLAIR.
Diffusion sequences show very bright signal on diffusion-weighted images ( a combination of restricted diffusion and T2 shine through). Postsurgical DWI follow-up is critical to detect any residual focus, which will be DWI bright.
Rarely, epidermoids may exhibit signal hyperintensity on unenhanced T1-weighted imaging, also known as “white epidermoids”.
Intra-axial neoplasm
A posterior fossa intra-axial neoplasm may invade laterally into the CPA.
An exophytic brainstem glioma or metastasis may invade into the CPA.
Medulloblastoma tends to occur in the midline in children, though lateral involvement of the cerebellar hemipsheres can be seen in older children or young adults.
Ependymoma may extend into the CPA by squeezing through the lateral 4th ventricular foramina (of Luschka).
Hemangioblastoma, associated with von Hippel-Lindau (VHL) disease, typically presents in the cerebellar hemispheres as a fluid-secreting tumor with a cyst and enhancing nodule. There are often prominent flow voids feeding the tumor.
Overview and anatomy of the sella, suprasellar region, and cavernous sinus
The pituitary gland is formed from Rathke’s puch, which is superior invagination from the primitive oral cavity. The pituitary gland sits in the sella turcica, a cup-shaped depression of the basisphenoid bone. The pituitary is composed of an anterior and a posterior lobe. (Rathke’s pouch closes off to form a vesicle that involutes. Sometimes, the involution is incomplete and a cleft can be left behind, which may give rise to craniopharyngioma or Rathke’s cleft cyst.
The anterior lobe of the pituitary produces and secretes endocrine hormones, including growth hormone, ACTH, prolactin, TSH, FSH, and LH.
The posterior lobe of the pituitary is derived from neuroectoderm and is composed of axons from the hypothalums, through which vasopressin and oxytocin are transported.
The pituitray gland has a wide rage of normal morphology, depending on patient age, sex, and hormonal/pregnancy status. The gland may be convex superiorly in adolescent or pregnant females. The normal posterior pituitary is hyperintense on T1-weighted MRI and is called the “posterior pituitary bright spot”, best seen on sagittal images.
The empty sella is a normal variant when seen in isolation. An empty sella is partially filled with CSF, with the gland flattened against the floor of the sella. Empty sella is also a component of the constellation of findings in pseudotumor cerebri. Pseudotumor cerebri, also known as idiopathic inctranial hypertension, is a syndrome associated with elevated CSF pressure, visual changes, and headaches that is typically seen in obese black females. Imaging findings include empty sella, enlargement of Meckel’s cave, and optic disc protrusion into the posterior globes. The ventricles are normal in size or slightly reduced in caliber. The sigmoid or transverse sinus may be stenotic.
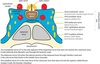
Approach to a sellar/parasellar mass
The first step in evaluation of a sellar region mass is to determine if the mass is intrinsic to the pituitary or if the mass represents an adjacent extra-pituitary lesion.
The differential for an intrinsic pituitary mass is rather limited and includes pituitary adenoma (by far the most common intrinsic pituitary mass), Rathke’s cleft cyst, and hypophysitis (inflammation of the pituitary). Craniopharyngioma may rarely occur in the sella, but essentially never occurs within the pituitary gland itself.
Intrinsic Pituitary Mass
A pituitary microadenoma is a pituitary adenoma <10 mm in size. Patients seek medical attention due to symptoms of hormone excess, not mass effect.
Most microadenomas are hypoenhancing relative to the pituitary, although ACTH-secreting adenomas may be hyperenhancing.
A macroadenoma is defined as an adenoma > 10 mm in size. Patients usually present with mass effect (e.g., compression of the optic chiasm) rather than endocrine dysfunction.
The bony sella is often enlarged. Macroadenomas may encase the carotid, but tend not to narrow it. In contrast, meningiomas or metastases can narrow the carotid. Pituitary macroadenoma may bleedafter medical treatment, producing a complex MRI appearance. Intrat-tumoral hemorrhage is distinct from pituitary apoplexy. Pituitary apoplexy is a clinical syndrome of severe headache and endocrine dysfunction caused by hemorrhage into an otherwise normal pituitary.
Lymphocytic hypophysitis is an autoimmune inflammatory disorder usually seen in peripartum women that may affect the pituitary and infundibulum. It presents with diabetes insipidus, headache, visualy impairment, and endocrine dysfunction.
MRI shows thickening and intense enhancement of the pituitary stalk,usually with enlargement of the pituitary gland that may appear similar to a macroadenoma.
Lymphocytic hypophysitis responds to steroid therapy.
Granulomatous inflammation of the pituitary and infundibulum can be secondary to sarcoidosis, Wegener granulomatosis, tuberculosis, and Langerhans cell histiocytosis (LCH). LCH hypophysitis is a dease of children. In all causes, imaging is dientical to lymphocytic hypohysitis.
Rathke’s cleft cyst may be limited to the pituitary gland but it is more commonly seen extrinsic to the pituitary.
Suprasellar Mass
The differntial diagnosis for an extra-pituitary lesion is broad, but the imaging findings together with clues about the patient’s age and clinical presentation can usually narrow the differential diagnosis to a few entities.
The most common suprasellar lesion in a child is craniopharyngioma, while the most common suprasellar lesion in an adult is a pituitary macroadenoma that has extended superiorly.
The SATCHMO mnemonic may be helpful to remember the spectrum of extra-pituitary masses; however, the order of the entities is NOT based on frequency of occurence. (Sarcoidosis/Suprasellar extension of an adenoma, Aneurysm, Teratoma (dermoid cyst)/Tolosa Hunt, Craniopharyngioma/Cleft cyst (Rathke’s), Hypothalamic glioma (adults)/Hypothalamic hamartoma (children), Optic nerve glioma)
Craniopharyngioma
Craniopharyngioma is themost common suprasellar lesion of childhood, arising from squamous epithelial remnants of Rathke’s pouch that produce keratin.
Craniopharyngioma occurs in a bimodal age distribution. The majority of cases are lesions of childhood, but craniopharyngioma may occur uncommonly in late middle age.
Most involve both the sella and suprasellar regions. Although craniopharyngioma may rarely involve only the sella, it is almost always separate from the pituitary gland.
Craniopharyngioma has potential for enamel production and almost always calcifies. The characteristic intracystic machine-oil seen on gross examination is composed of desquamated squamous epithelium, keratin, and cholesterol.
MRI shows a complex cystic mass containing protein or blood products (hyperintense on T1-weighted images).
There is avid enhancement of the solid elements and cyst walls.
In contrast to Rathke’s cleft cyst, craniopharyngioma almost always enhances, is almost always calcified, and is almost always separate from the pituitary.
Rathke’s cleft cyst
Similar to craniopharyngioma, Rathke’s cleft cyst is also a remnant of the embryologic Rathke’s pouch, the precursor of the anterior lobe of the pituitary gland. In contrast to craniopharyngioma, Rathke’s cleft cyst is made of simple columnar or cuboidal epithelium.
While craniopharyngioma is the most common suprasellar lesion of childhood, Rathke’s cleft cyst is typically seen in middle-aged adults, twice as commonly in females.
Rathke’s cleft cyst is reportedly very common in autopsy studes (up to 22% incidence), but clinically is usually asymptomatic or discovered incidentally.
Imaging appearance is dependent on the protein content of the cyst. The intra-cystic fluid may be isointense to CSF if low protein and hyperintense on T1-weighted images if high protein. High protein content may cause incomplete nulling of the intracystic fluid on FLAIR>
The claw sign represents enhancing pituitary tissue completely wrapped around the cyst.
It is usually possible to distinguish craniopharyngioma from Rathke’s cleft cyst. Unlike craniopharyngioma, Rathke’s cleft cyst does not enhance (although rim enhancement is often seen) and does not calcify. Rathke’s cleft cyst may occasionally be inseparable from the pituitary, but craniopharyngioma is nearly always distinct.
Meningioma (Suprasellar)
Meningioma is the second most common suprasellar tumor in adults. Most common in middle-aged females, it typically presents with visual loss due to optic pathway involvement. There are several dural reflections in the region of the sella from which a meningioma may arise, including the tuberculum sella, clinoid process, planum sphenoidale, and sphenoid wing.
Imaging shows a isointense signal on T1-weighted images, variable signal on T2-weighted images, and uniform, intense contrast enhancement. There is often an enhancing dural tail. Meningioma may cause adjacent hyperostosis due to vasoactive factors.
An important imaging finding of a parasellar meningioma is the tendency to encase and narrow the cavernous or supraclioid internal carotid artery.
In contrast to pituitary adenoma, the sella is usually normal and the pituitary can be identified separately.
Astrocytoma (optic pathway glioma)
An astrocytoma involving the visual pathway (optic nerve, optic chiasm, and optic tract) is the second most common suprasellar mass in children (craniopharyngioma is most common). A substantial minority of patients with optic pathway glioma have neurofibromatosis type 1. In contrast to the low-grade tumor of childhood, optic glioma is an aggressive tumor when it occurs in adults.
Tumors are isointense on T1-weighted images, hyperintense on T2-weighted images, and usually enhance.
Germinoma
The most common intracranial germ cell tumor is a germinoma, of which 80% arise in the pineal region and 20% arise in the parasellar region. Germinomas are primarily seen in children and adolescents.
Imaging shows a homogenous, intensely enhancing midline mass. The mass is hypointense on T2-weighted images and dark on ADC map due to hypercellularity.
Epidermoid and dermoid cysts
Epidermoid and dermoid cysts are congenital benign inclusion cysts.
Epidermoids occur most commonly in middle-aged adults in the cerebellopontine angle, but can be seen less commonly in the parasellar region. Epidermoids follow CSF signal on T1- and T2-weighted images. In contrast to a simple arachnoid cyst, epidermoid is hyperintense on FLAIR and diffusion sequences show restricted diffusion.
Dermoids are most common in young adult males in the posterior fossa, but may occasionally occur in the parasellar region. They may contain intracystic fat which can cause chemical meningitis or ventriculitis with rupture.
Hypothalamic Hamartoma
Hypothalamic hamartoma is not a true neoplasm, but represents ectopic hypothalamic neural tissue. It is a rare lesion of childhood that classically presents with precocious puberty and gleastic seizures (laughing spells)
Hypothalamic hamartoma characteristically appears as a sessile mass between the pituitary stalk and the mammillary bodies.
Hypothalamic hamartoma does not enhance and is isointese to gray matter.
Aneursym
A saccular supraclinoid internal carotid artery aneurysm may mimic a suprasellar tumor.
Although parasellar aneurysms are relatively uncommon, it is essential never to biopsy a mass that may represent an aneursym.
Pulsation artifact may be present on conventional MRI sequences. CTA or MRA would be diagnostic.
Metastasis (suprasellar) and Lymphoma
Breast cancer is by far the most common lesion to metastasize to the parasellar region.
Parasellar lymphoma is rare but may occur in older adults.
Differential Diagnosis of a suprasellar mass is highly dependent on age

Intrinsic Pineal Mass
The pineal gland is located in the midline at the level of the midbrain. It is situated between the thalami at the posterior aspect of the third ventricle. The internal cerebral veins and vein of Galen are located superior and posterior to the pineal gland, repsectively.
The principal neuronal cell of the pineal gland is the pinealocyte, which is a modified retinal neuronal cell that is innervated by the sympathetic plexus originating in the retina. The pineal gland releases melatonin, which modulates the sleep/wake cycle. The pineal gland does not have a blood brain barrier.
A mass lesion in the pineal region may cause compression of the midbrain, compression of the cerebral aqueduct of Sylvius, or compression of the tectal plate. Compression of the tectal plate produces Parinaud syndrome, which is the inability to look up (upward gaze paralysis), pupillary light dissociation, and nystagmus.
The first step in evaluating a pineal mass is to determine if the lesion is arising from the pineal gland itself or from an adjacent structure.
A mass of the pineal gland is extra-axial.
Tumors of pineal cell origin tend to lift the internal cerebral veins, while tentorial meningiomas tend to depress the internal cerebral veins. The relationship of any pineal region mass to the internal cerebral veins is key for surgical planning and approach.
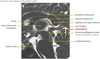
Germ cell tumor
Extragonadal germ cell tumors can be found in the pineal gland as well as other intracranial and extracranial midline locations including the suprasellar region, mediastinum, and sacrococcygeal region. Extragonadal germ cell tumors are thought to be due to aberrant migration of totipotent germ cells during early embryogenesis.
Germinoma and teratoma are germ cell tumors, which are the most common and second most commmon pineal region tumors, respectively.
Germinoma (extra-gonadal seminoma) is the most commonn pineal region tumor and has a peak incidence in the second decade of life (age 10-19). Pineal germinoma is seen much more commonly in males, but suprasellar germinoa does not show a gender predilection. Germinoma is a highly cellular, avidly enhancing tumor that is slightly hyperdense on CT, isointense on T1- and T2-weighted images, and is dark on ADC map.
Germinoma characteristically “engulfs” the pineal gland and promotes its calcification, resulting in a central area of calcification.
Imaging of the entire neuraxis is recommended as leptomeningeal deposits can occur.
Treatment is readiotherapy, with excellent prognosis.
Pineal germinoma may present with a synchronous suprasellar germ cell tumor.
Teratoma is the second most common pineal region tumor and confers a worse prognosis than germinoma. Teratoma has a heterogenous imaging appearance. Intralesional fat is suggestive of teratoma. Teratoma is prone to hemorrhage and coarse calcification.
Pineal cyst
Pineal cysts are seen commonly on MRI and have a prevalence as high as 40% on autopsy series. They are more common in women. Most pineal cysts are less than 1 cm and asymptomatic, but may cause symptoms due to mass effect.
Very few pineal cysts grow and follow-up of small pineal cysts is not routinely recommended.
Pineal cysts are usually not entirely simple. Most cysts do not fully suppress on FLAIR. Most cysts do display some peripheral enhancement, and rim calcification can be seen about 25%of the time.
A differential consideration is a pineocytoma, which would demonstrate internal enhancement and may have cystic components. However, a truly cystic pineocytoma is considered very rare. A potential pitfall is that if imaging is delayed after contrast administration (by greater than 60 minutes), gadolinium may diffuse into the cyst, causing it to appear solid. In rare cases, it may not be possible to differentiate a hemorrhagic pineal cyst from a pineocytoma.
Pineocytoma
A pineocytoma is a low-grade (WHO grade I or II), slow-growing pinealocyte tumor.
Any solid component should enhance. Pineocytoma may feature cystic change, however, which can make differentiation from a pineal cyst difficult.
Pineoblastoma
Pineoblastoma is a highly malignant WHO grade IV tumor of young children, of the same primitive neuroectodermal tumor (PNET) type as medulloblastoma.
The term trilateral retinoblastoma is used when bilateral retinoblastomas are also present (both the retina and pineal gland are light-sensing organs). The sella is additionally involved in quadrilateral retinoblastoma.
Pineoblastoma often presents with obstructive hydrocephalus. Pineoblastoma characteristically appears as a poorly defined pineal mass that may invade into adjacent structures. High cellularity causes restricted diffusion.
In contrast to germinoma, which “engulfs” and induces calcification of the pineal gland, pineoblastoma peripherally calcifies in a pattern that has been likened to “exploded” calcification.
Pineoblastoma has a propensity for leptomeningeal metastasis and CSF seeding.
Pineal Metastases
Due to the lackof a blood brain barrier, metastasis to the pineal gland occur relatively commonly, but rarely in the absence of a known malignancy.
Leptomeningeal disease is present in two-thirds of patients with pineal metastasis.
Pineal region mass
Gliomas (most commonly astrocytomas) of varying grademay occur in adjacent intra-axial structure such as the tectum, midbrain, or splenium of the corpus callosum.
Despite the name, a vein of Galen “aneurysm” is not a true aneurysm. Instead, it represents dilation of the vein of Galen due to an arteriovenous fistula between the anterior or posterior circulationand the venous plexus leading to the vein of Galen.
The tentorial apex, adjacent to the pineal gland, is a characteristic location for meningioma.
As previously discussed, a tentorial meningioma tends to depress the internal cerebral veins, in contrast to a pineal-based mass, which typically elevates the internal cerebral veins.
A lipoma of the quadrigeminal plate is a rare lesion that can be seen in isolation or associated with agenesis or hypoplasia of the corpus callosum.
The quadrigeminal plate is another name for the tectum.
Extra-axial Hemorrhage
Acute extra-axial hemorrhage (subarachnoid, epidural, or subdural in location) is usually hyperattenuating when imaged by CT; however, blood must clot in order to be hyperattenuating. Hyperacute unclotted blood (and clotted blood in a patient with severe anemia) may be close towater attenuation on CT.
Subarachnoid hemorrhage (SAH)
Trauma is the most common cause of subarachnoid hemorrhage (SAH), while aneurysm rupture is the most common cause of non-traumatic SAH.
Traumatic SAH tends to occur contralateral to the side of direct impact, most often in the superficial cerebral sulci.
Epidural Hematoma
An arterial epidural hematoma is an extra-axial collection of blood external to the dura, classically caused by fracture of the squamous portion of the temporal bone and resultant tearing of the middle meningeal artery.
An arterial epidural hematoma has a lentiform shape and does not cross the cranial sutures, where the dura is tightly adherent to the cranium.
The swirl sign describes mixed high and low attenuation blood within the hematoma and suggests active bleeding. The low attenuation bloodis hyperacute unclotted blood while the high attenuation blood is already clotted.
A large epidural hematoma is a surgical emergency due to mass effect and risk of herniation, although small epidural hematomas can be conservatively managed with serial imaging.
Venous epidural hematomas are far less common than arterial epidurals and are due to laceration of the dural sinuses, usually occurring in the posterior fossa in children.
Subdural Hematoma
A subdural hematoma is a crescenteric extra-axial collection of blood located beneath the dura. Since it is underneath the dura, the hematoma can extend across the cranial sutures. Subdural hematomas often extend along the surfaces of the falx cerebri and tentorium cerebelli.
Subdural hematomas typically result from tearing of cerebral veins. Patients with atrophic involutional changes are at increased risk of subdural hematoma with even minor trauma, as the cerebral veins stretch to traverse the enlargd CSF spaces.
A particular danger is a subdural hematoma in a patient with a ventricular shunt because the shunted ventricular system does not function as a natural tamponade.
An isodense subdural hematoma is isoattenuating to gray matter. This occurs in the subacute phase approximately 1-3 weeks after the initial injury. Three important clues alerting to the presence of an isodense subdural are increased mass effect, white matter buckling, and an apparently thickened cortex.
Intraventricular hemorrhage
Intraventricular hemorrhage can occur due to tearing of the subependymal veins or from direct extension of subarachnoid or intraparenchymal hematoma.
Patients with intraventricular hemorrhage are at increased risk of developing noncommunicating hydrocephalus due to ependymal scarring, which may obstruct the cerebral aqueduct.
Intra-axial injury
The coup/contrecoup mechanism of brain trauma describes the propensity for brain to be injured both at the initial site of impact and 180° opposite the impact site, due to secondary impaction against the cranial vault.
Cortical contusion
A cortical contusion is caused by traumatic contact of the cortical surface of the brain against the rough inner table of the skull. Contusions affect the gyral crests and can occur in a coup or a contrecoup location.
A subacute cortical contusion may demonstrate ring enhancement and should be considered in the differential of a ring enhancing lesion if there is a history of trauma. Enhancement may continue into the chronic stage.
A chronic contusion appears as ecephalomalacia on CT. MRI is more specific, showing peripheral hemosiderin deposition as hypointense on T2-weighted images and blooming artifact on gradient echo sequences.
Intraperanchymal hematoma
Traumatic intraparenchymal hematoma can occur in various locatios, ranging from cortical contusion to basal ganlgia hemorrhage (due to shearing of lenticulostriate vessels).
Similar to a cortical contusion, a subacute intraparenchymal hematoma may show ring enhancement.
Diffuse axonal injury (DAI)/ Traumatic axonal injury (TAI)
Diffuse axonal injury (DAI) is the result of a shear-strain deformation of the brain.
The term traumatic axonal injury (TAI) has recently been introduced as this injury pattern is thought to be multifocal rather than diffuse; however, this text will use the term DAI, as that is the more common term.
DAI is caused by rotational deceleratin and subsequent reacceleration force that exceeds the limited elastic capacity of the axons.
The most common locations of DAI include the gray-white matter junction,the corpus callosum, and the dorsolateral midbrain. The higher the grade, the worse the prognosis. Grade I DAI involves only the gray-white matter junctions. Grade II DAI involves the corpus callosum. Grade III (most severe) DAI involves the dorsolateral midbrain.
CT is relatively insensitive for detection of DAI, although hemorrhagic DAI may show tiny foci of high attenuation in the affected regions.
MRI is much more sensitive to detect DAI, although detection relies on multiple sequences, including FLAIR, GRE, and DWI
GRE is extremely sensitive for hemorrhagic axonal injry; however, not all DAI is hemorrhagic. FLAIR is most sensitive for nonenhacing DAI. Diffusion sequences show restricted diffusion in acute DAI due to cytotoxic edema and cell swelling.
Zygomaticomaxillary complex fractures
Commonly but incorrectly know as the tripod fracture, a zygomaticomaxillary complex (ZMC) fracture causes a floating zygoma by disrupting all four of the zygomatic articulations.
The zygoma normally articulates with the frontal, maxillary, temporal, and sphenoid bones via the zygomaticofrontal, zygomaticomaxillary, zygomaticotemporal, and zygomaticosphenoid articulations.
A ZMC fracture causes disruption of the zygomatic articulations by fractures through the follow structures:
Lateral orbital rim fracture: Zygomaticofrontal disruption
Inferior orbital rim fracture: Zygomaticomaxillary disruption.
Zygomatic arch fracture: Zygomaticotemporal disruption.
Lateral orbital wall: Zygomaticosphenoid disruption.
Le Fort Fractures
The Le Fort classification describes a predictable pattern of midface fractures, all of which disrupt the pterygomaxillary buttress and cause detachment of the maxilla from the skull base. All Le Fort fractures are defined by fractures though the pterygoid plates.
Le Fort I (floating plate) detaches the maxillary alveolus from the skull base.
Le Fort II dissociates the central midface from the skull, causing the nose and hard palate to be moved as a single unit.
Le Fort III represents a complete midface dissocation.
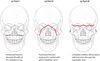
Central sulcus
The central sulcus separates the motor strip (frontal lobe) from the sensory cortex (parietal lobe).
To find the central sulcus, follow the cingulate sulcus posteriorly on a slightly off-midline sagittal (left images above). The cingulate sulcus connects to the marginal ramus. Directly anterior to the marginal ramus is the paracentral lobule, which contains both the motor strip and the sensory cortex.
On an axial image, the central sulcus forms a characteristic upside down omega. The corresponding region of motor strip, just anterior to the omega, controls the hand.

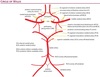
Internal Carotid Artery
Cervical (C1): Doe not branch within the neck
Petrous (C2): Fixed to bone as the ICA enters the skull base, so a cervical carotid dissection is unlikely to extend intracranially.
Lacerum (C3): No branches.
Cavernous (C4): The meningohypophyseal trunk arises from the cavernous carotid to supply the pituitary, tentorium, and dura of the clivus. The inferolateral trunk also arises from C4 to supply the 3rd, 4th, and 6th cranial nerves, as well as the trigeminal ganglion.
Clinoid segment (C5): The carotid rings are two dural rings that mark the proximal and distal portions of the clinoid segment of the ICA. The carotid rings prevent an inferiorly located aneurysm from causing intracranial subarachnoid hemorrhage with rupture.
Supraclinoid (C6-C7): Gives off several key arteries:
The opthalmic artery supplies the optic nerve. It takes off just distal to the distal carotid ring in 90% of cases and can be used as a landmark for the distal ring. Aneursyms located superior to this ring can result in subarachnoid hemorrhage. Given this risk, these aneursyms are treated more aggressively than aneurysms located proximal to the distal dural ring, which are contained.
The posterior communicating artery (P-comm) is an anastomosis to the posterior circulation. A fetal posterior cerebral artery (PCA) is a variant supplied entirely by the ipsilateral ICA via an enlarged P-comm.
The anterior choroidal artery supplies several critical structures, despite its small size. It supplies the optic chiasm, hippocampus, and posterior limb of the internal capsule.
Circle of Willis
The A1 segment of the anterior cerebral artery (ACA) travels above the optic nerves and give off the recurrent artery of Huebner, which supplies the caudate head and anterior limb of the internal capsule. The A1 segment also gives rise to the medial lenticulostriate perforater vessels, which supply the medial basal ganglia.
Just outside the circle of Willis, the middle cerebral artery (MCA) gives rise to the lateral lenticulostriate perforator vessels to supply the lateral basal ganglia include the lateral putamen, external capsule, and the posterior limb of the internal capsule.
The posterior communicating artery (P-comm) travels between the optic tract and the 3rd cranial nerve, giving off anterior thalamoperforator vessels. A P-comm aneurysm may cause cranial nerve III palsy due to local mass effect.
The posterior cerebral artery (PCA) gives off thalamoperforators to supply the thalamus. Artery of Percheron is a variant where there is a dominant thalamic perforator supplying the ventromedial thalami bilaterally and the rostral midbrain, arising from a P1 PCA segment. An artery of Percheron infarct will result in bilateral ventromedial thalamic infarction, with or without midbrain infarction (the infarct may be V shaped if the midbrain is involved). Deep venous thrombosis may also result in bilateral thalamic infarcts.
The anterior choroidal artery is the most distal branch of the internal carotid artery. It supplies the optic chiasm, hippocampus, and posterior limb of the internal capsule.
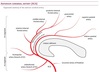
Middle cerebral artery (MCA)
Although the transition form M1 to M2 is technically defined as the upward point of deflection into the sylvian fissure, in practical terms, the pre-bifurcation MCA is often called M1 and the post-bifurcation MCA is called M2.

Anterior Cerebral Artery (ACA)
The recurrent artery of Huebner arises most commonly from the A1 segment of the ACA, proximal to the anterior communicating artery. The recurrent artery of Heubner supplies the head of the caudate and the anterior limb of the internal capsule.
Persistent Carotid-Basilar Connections
A number of carotid to basilar connections are formed during embyrogenesis. These fetal anterior-posterior circulation connections normally regress before birth.
Occasionally, a fetal carotid-basilar connection may persist after birth. Each anomalous connection is named for the structures adjacent to its course in the head and neck.
A persistent trigeminal artery is the most common persistent carotid-basilar connection and has an association with aneurysms.
The persistent trigeminal artery courses adjacent to the trigeminal nerve. Angiography shows a charcteristic trident or tau sign on the laterla view due to the artery’s branching structure.
Saltzman type I connects to the basilar artery while Saltzman type II connects to the superior cerebellar artery.
The otic, hypoglossal, and proatlantal intersegmental arteries are rare persistent carotid-basilar connections.
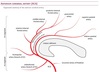
Arterial Territories
Stroke Imaging and guidelines
The goal of stroke imaign is to determine who would benefit from therapy.
The goal of stroke therapy is to restore perfusion to the brain.
In the appropriate patients, intravenous or intra-arterial thrombolysis performed with tissue plasminogen activator (tPA) can have near-miraculous results. However, there is a grave risk of fatal hemorrhage if patients are inappropriately selected for therapy. The exact exclusion criteria for administration of thrombolytic therapy varies among institutions.
The American Heart Association (AHA) published guidelines for early management of adults with ischemic stroke (Stroke, 2007) and established recommendations for imaging and treatment of acute stroke.
Imaging of the brain is recommended before thrombolytic therapy is administered and the imaging study should be interpreted by a physician with expertise in reading brain studies. Initial imaging in suspected acute stroke is usually noncontrast CT, for the primary purpose of excluding hemorrhage. However, some authors assert that MRI is equally sensitive for detecting hemorrhage using GRE sequences.
Advanced imaging (with CT or MR) includes vascular imaging, diffuse-weighted imaging, and perfusion imaging. These advanced imaging studies may provide additional information, but are not required before the initiation of thrombolytic therapy. In fact, advanced imaging should not delay treatment.
Per AHA guidelines, the only CT finding that absolutely precludes intravenous tPA within 3 hours of onset of stroke is the presence of hemorrhage. Some authors advocate for extending the window for tPA administration to 4.5 hours from stroke onset, although this expanded window is not discussed in the AHA guidelines.
Intra-arterial thrombolysis may be performed for an MCA thrombus within 6 hours of stroke onset in patients who are not candidates for intravenous thrombolysis. Subsequent to the development of the AHA guidelines, some authors recommend extending the window for intra-arterial treatment of anterior circulation stroke to 8 hours. Similarly, many authors argue for no time limit for intraarterial tPA for posterior circulation infarction because these strokes can be catastrophic if untreated.
Some institutions add additional exclusion criteria for administration of intravenous tPA, although these additional criteria are not a part of the AHA guidelines:
Individuals with a large (greater than 1/3 MCA territory) infarct may be excuded from IV tPA.
Occlusion of the distal internal carotid artery and proximal MCA and ACA (a T-shaped occlusion) may preclude treatment with IV tPA.
Absence of a penumbra of salvageable brain that represents at least 20% of the region of abnormal perfusion may preclude treatment with IV tPA.
Perfusion stroke imaging
The role of perfusion CT or MRI in the management of acute stroke is evolving and remains controversial. The theoretical goal of perfusion imaging is to characterize the ischemic penumbra, which is the area of vulnerable brain adjacent to the infarct core that may also become infarcted without intervention. Currently, no clinical guidelines exist regarding the implementation of perfusion imaging.
The penumbra does receive some perfusion, but at a reduced rate compared to normal brain. Perfusion of the penumbra is < 20 mL/100 g tissue per minute in physiologic studies, compared to ~60 mL/100 g tissue per minute for normal gray matter. Such a low rate of perfusion causes cellular dysfunction and produces a neurologic defict, which may be restored with therapy.
The infarct core is usually dead tissue, which genrally cannot recover even after therapy.
Acute stroke: Noncontrast CT imaging
Noncontrast CT is the initial test of choice for evaluation of hyperacute infarct when the patient presents within the IV tPA time window (3 hours, or 4.5 hours at some institutions).
The main purpose of a noncontrast CT is to exclude patients who would be harmed by thrombolytic therapy as discussed above, most importantly to exclude those with hemorrhage.
Noncontrast CT in the hyperacute stage is relativley insensitive to detect early infarction compared to MRI. Sublte loss of gray-white differentiation in the insula or basal ganglia may be present on CT, thought to be due to decreased cerebral blood volume.
The insular ribbon sign describes the loss of gray-white differentiation in the insula. The gray-white junction becomes most conspicuous at very narrow stroke windows (window 30/level 30)
Obscuration of the lentiform nucleus (putamen and globus pallidus) is caused by loss of gray-white differentiation at the border of the lentiform nucleus and the posterior limb of the internal capsule.
The hyperdense artery sign describes direct visualization of the acute intravascular thrombus, most commonly seen in the MCA. The hyperdense artery sign is specific for ischemia when seen, but relatively insensitive (seen in approximately one third of cases). Some authors suggest that the prsence of the hyperdense artery sign portends a worse prognosis.
Acute stroke: MR Imaging
Detailed MRI imaging of the multiple temporal stages of stroke is discussed on the following pages. For the initial evaluation, diffusion sequences can detect acute infarction with high sensitivity within minutes of symptom onset. DWI is more sensitive than FLAIR in the detection of hyperacute stroke.
Evolution of Infarction
Hyperacute infarct (0-6 hours)
- Within minutes of critical ischemia, the sodium-potassium ATPase pump that maintains the normal low intracellular sodium concentration fails. Sodium and water diffuse into cells, laeding to cell swelling and cytotoxic edema.
- Calcium also diffuses into cells, which triggers cascades that contribue to cell lysis.
- By far the most sensitive imaging modality for detection of hyperacute infarct is MRI diffusion-weighted imaging (DWI). DWI hyperintensity and ADC map hypointensity reflect reduced diffusivity, which can be seen within minutes of the ictus.
- Diffusion is reduced in an acute infarct by two factors:
- 1) Shift from extracellular to intracellular water due to Na/K ATPase pump failure.
- 2) Increased viscosity of infarcted brain due to cell lysis and increased extracellular protein.
- FLAIR may be normal. Subtle hyperintensity may be seen on FLAIR images in the hyperacute stage. These changes are seen less than two thirds of the time within the first six hours.
- Perfusion shows decreased cerebral blood volume of the infarct core, with or without a surrounding region of decreased cerebral blood flow, which represents the penumbra.
Acute infarct (6 hours - 72 hours)
- The acute infarct phase is characterized by increase in vasogenic edema and mass effect.
- Damaged vascular endothelial cells cause leakage of extracellular fluid and increase the risk of hemorrhage.
- On imaging, there is increased sulcal effacement and mass effect. The mass effect peaks at 3-4 days, which is an overlap time between the acute and early subacute phases.
- MRI shows hyperintenisty of the infarct coreon T2-weighted images, best seen on FLAIR. The FLAIR abnormality is usually confined to the gray matter. DWI continues to show restricted diffusion.
- There may be some arterial enhancement, due to increased collateral flow.
- Perfusion images most commonly show increase in size of the infarct core with resultant decrease in size of the penumbra.
Early subacute infarct (1.5 days - 5 days)
- In the early subacute phase, blood flow to the affected brain is re-established by leptomeningeal collaterals and ingrowth of new vessels into the region of infarction.
- The new vessels have an incomplete blood brain barrier, causing a continued increase in vasogenic edema and mass effect, which peaks at 3-4 days.
- MR imaging shows marked hyperintensity on T2-weighted images involving both gray and white matter (in contrast to the acute stage, which usually involves just the gray matter).
- The ADC map becomes less dark or even resolves if there is extensive edema; however, the DWI images typically remain bright due to underlying T2 shine through.
- Perfusion imaging shows continued expansion of the infarct core and further reduction in the ischemic penumbra.
Late subacute infarct (5 days - 2 weeks)
- The subacute phase is characterized by resolution of vasogenic edema and reduction in mass effect.
- A key imaging finding is gyriform enhancement, which may occasionally be confused for a neoplasm. Unlike a tumor, however, a subacute infarction will not typically demonstrate both mass effect and enhancement simultaneously. Enhancement can be seen from approximately 6 days to 6 weeks after the initial infarct. (The enhancement of a subacute infarct has also been described by the “2-2-2” rule, which states the enhancement begins at 2 days, peaks at 2 weeks, and disappears by 2 months.
- DWI may remain bright due to T2 shine through, although the ADC map will either return to normal or show increased diffusivity.
Chronic Infarct
- In the chronic stage of infarction, cellular debris and dead brain tissue are removed by macrophages and replaced by cystic encephalomalacia and gliosis.
- Infarct involvement of the corticospinal tract may cause mass effect, mild hyperintensity on T2-weighted images, and eventual atrophy of the ipsilateral cerebral peduncle and ventral pons due to Wallerian degeneration. These changes can first be seen int eh subacute phase, with atrophy being the predominant feature in the chronic stage.
- DWI has usually returned to normal in the chronic stage.
- Occasionally cortical laminar necrosis is a histologic finding characterized by deposition of lipid-laden macrophages after ischemia that manifests on imaging as hyperintensity on both T1- and T2-weighted images.

Arteriovenous Malformation (AVM)
An arteriovenous malformation (AVM) is a congenital high-flow vascular malformation consisting of directly connecting arteris and veins without an intervening capillary bed. AVM occurs intra-axiallyand 85% are supratentorial. AVM usually presents with seizures or bleeding (usually parenchymal hemorrhage, rarely subarachnoid). Aneurysms of the feeding arteries or intra-nidal arteries are often seen, which predispose to bleeding.
The Spetzler-Martin scale helps to evaluate surgical risk for AVM resection. A large AVM draining to a deep vein in eloquent cortex is high risk, while a small AVM draining to a superficial vein in non eloquent cortex is low risk.
On imaging, AVM is characterized by a vascular nidues (“nest”) containing numerous serpentine vessels that appear as black flow-voids on MRI. There are usually adjacent changes to the adjacent brain including gliosis (T2 prolongation), dystrophic calcification, and blood products (blooming on T2* gradient imaging). The gliosis/encephalomalacia or mineralization seen in the adjacent brain is due to alteration in vascular flow from the AVM.
AVM replaces rather than displaces brain. It causes minimal mass effect.
Uncommonly, a bleeding AVM may be angiographically occult if the malformed vessels are compressed by the acute hematoma.
Factors that increase bleeding risk that are detectable by imaging include intra-nidal aneurysm, venous ectasia, venous stenosis, deep venous drainage, and posterior fossa location.
Treatment can be with embolization, stererotactic radiation, or surgical resection.
Vein of Galen malformation is a type of vascular malformation characterized by arteriovenous fistulae from the thalamoperforator branches into the deep venous system. The enlarged vein is actually an enlarged median prosencephalic vein. In childhood, a vein of Galen malformation is the most common extracardiac cause of high output cardiac failure. Vein of Galen malformation may also be seen in adults, but clinically would be either asymptomatic or may be the cause of Parinaud syndrome due to mass effect in the pineal region.
Dural arteriovenous fistula (dAVF)
Dural arteriovenous fistulas are a complex group of high-flow lesions characterized by arteriovenous shunts between the meningeal arterioles and dural venules.
The primary prognostic feature is the presence and degree of cortical venous drainage. The Cognard classification I through IV describes lesions with progressively increased risk of bleeding. Type V is reserved for spinal dAVFs.
Type I: No cortical venous drainage. Lowest risk of bleeding.
Type IIA: Reflux into dural sinus but not cortical veins.
Type IIB: Refulx into cortical veins: 10-20% hemorrhage rate.
Type III: Direct cortical venous drainage: 40% hemorrhage rate.
Type IV: Direct cortical venous drainage with venous ectasia: 66% hemorrhage rate.
Type V: Spinal venous drainage. May cause myelopathy.
Carotid-cavernous fistual (CCF) is a subtype of dAVF that is often caused by trauma with resultant fistual between the cavernous carotid artery and the cavernous sinus. Enlargement of the superior orbital vein and shunting within the cavernous sinus can lead to eye symptoms, such as proptosis and cranial nerve palsy.
Cavernous Malformation (cavernoma)
A cavernous malformation (also called a cavernoma) is a vascular hamartoma with a very small but definite bleeding risk. The clinical course of a cavernous malformation is variable and the lesion may cause seizures even in the absence of significant hemorrhage.
Cavernous malformation is often associated with an adjacent developmental venous anomaly (DVA). There is increased risk of bleeding if a DVA is present. However, the DVA itself does not have any bleeding risk.
When multiple, cavernous malformations represent an inerited disorder called familial cavernomatosis.
Cavernous malformations can be induced by radiation treatment to the brain.
Noncontrast CT shows a well-circumscribed rounded hyperattenuating lesion. The hyperattenuation is due to microcalcification within the cavernoma.
MRI shows characteristic “popcorn-like” appearance of lobular mixed signal on T1- and T2-weighted images from blood products of various ages. There is a peripheral rim of hemosiderin which is dark on GRE and T2-weighted image. There is typically no enhancement, but intense enhancement may be seen with a long delay after contrast administration. Cavernous malformations may range in size from tiny (single focus of susceptibility artifact) to giant.
Cavernous malformations are usually occult by vascular imaging (CTA or angiography).
Developmental venous anomaly (DVA), also called venous angioma
A developmental venous anomaly (DVA) is an abnormal vein that provides functional venous drainage to normal brain.
DVA can usually only be seen on contrast-enhanced images, where it appears as a radially oriented vein with a characteristic caput medusa appearance.
A DVA is a Do Not Touch lesion. If resected, the patient will suffer a debilitating venous infarct. The DVA must be preserved if an adjacent cavernous malformation is resected.
Capillary telangiectasia
A capillary telangiectasia is an asymptomatic vascular lesion composed of dilated capillaries with interspersed normal brain. A capillary telangiectasia is another Do Not Touch lesion.
Post-contrast MRI shows a faint, brush-stroke-like enhancing lesion in the brainstem or pons, without mass effect or surrounding edema. GRE may show blooming due to susceptibility.
Similar to cavernous malformation, capillary telangiectasia is angiographically occult.
Subarachnoid Hemorrhage (SAH)
Overall, the most common cause of subarachnoid hemorrhage (SAH) is trauma. Aneurysm rupture is by far the most common cause of non-traumatic subarachnoid hemorrhage. No cause of the subarachnoid hemorrhage is identified in up to 22% of cases.
Clinically, non-traumatic subarachnoid hemorrhage presents with thunderclap headache and meningismus.
Noncontrast CT is the initial imaging modality in suspected subarachnoid hemorrhage. On CT, subarachnoid blood appears as high attenuation within the subarachnoid space. High attenuation material in the subarachnoid space may be due to SAH (by far the most common cause), meningitis, leptomeningeal carcinomatosis, or prior intrathecal contrast administration.
Noncontrast CT is >95% sensitive for detecting subarachnoid hemorrhage within the first six hours, with sensitivity slowly decreasing to 50% by day 5. If clinical suspicion for subarachnoid hemorrhage is high with a negative CT scan, the standard of care is to perform a lumbar puncture to look for xanthochromia.
If SAH is present on imaging or lumbar puncture shows xanthochromia, catheter angiography is the gold standard to evaluate for the presence of an aneurysm. Several recent studies have shown, however, that CT angiography is equivalent to catheter angiography inthe search for a culprit aneurysm in cases of SAH.
On MRI, acute subarachnoid hemorrhage appears hyperintense on FLAIR and demonstrates susceptibility artifact on gradient sequences. The differential diagnosis for increased FLAIR signal in the subarachnoid space is similar to the differential for high attenuation subarachnoid material seen on CT, including SAH, meningitis, leptomeningeal carcinomatosis, and residual contrast material. Note that meningitis and carcinomatosis will typically show leptomeningeal enhancement in addition to the abnormal FLAIR signal. Recent oxygen or propofol administration will also cause increased subarachnoid FLAIR signal.
Distribution of Subarachnoid hemorrhage
The pattern of subarachnoid hemorrhage may provide a clue to the location of the ruptured aneurysm. However, multiple aneurysms are seen in up to 20% of patients with SAH, and subarachnoid blood may redistribute if the patient was found down.
Hemorrhage in the anterior interhemispheric fissure suggests an anterior communicating artery aneurysm (33% of intracranial aneurysms).
Hemorrhage in the suprasellar cistern suggests a posterior communicating artery aneurysm (also 33% of intracranial aneurysms). Rarely, P-comm aneurysm rupture can result in isolated subdural hemorrhage.
Hemorrhage in the sylvian fissure suggests a middle cerebral artery aneurysm (20% of intracranial aneurysms).
Hemorrhage in the perimesencephalic cistern suggests either a basilar tip aneurysm (5% of intracranial aneurysms), which has a high morbidity, or the relatively benign nonaneurysmal perimesencephalic subarachnoid hemorrhage (subsequently discussed).
Grading of subarachnoid hemorrhage
The Hunt and Hess score is the clincal grading scale for aneursymal subarachnoid hemorrhage and is based soley on symptoms, without imaging. Grade I is the lowest grade, with only a mild headache. Grade V is the most severe, with coma or extensor posturing.
The Fisher grade calssifies the CT appearance of SAH. Grade 1 is negative on CT; grades 2 and 3 are < 1 mm thick and > 1 mm thick, respectively, and grade 4 is diffuse SAH or intraventricular or parenchymal extension.
Complications of subarachnoid hemorrhage
Vasospasm is the most common cause of morbidity and mortality in patients who survive the intial episode of subarachnoid hemorrhage. The peak incidence of vasospasm occurs approximately 7 days after the initial ictus. Vasospasm may lead to stroke or hemorrhage.
Approximately 20-30% of patients with subarachnoid hemorrhage will develop acute hydrocephalus, due to obstruction of arachnoid granulations. Treatment is ventriculostomy.
Superficial siderosis is a condition caused by iron overload of pial membranes due to chronic or repeated subarachnoid bleeding. Clinically, patients with superficial hypointensity on T2-weighted images outlining the affected sulci.
Imaging workup includes cranial and spinal imaging to evaluate for a source of bleeding.
Perimesencephalic subarachnoid hemorrhage
Perimesencephalic subarachnoid hemorrhage is a type of nonaneurysmal subarachnoid hemorrhage that is a diagnosis of exclusion with a much better prognosis than hemorrhage due to a ruptured aneurysm.
The hemorrhage must be limited to the cisterns directly anterior to the midbrain. The standard of care is to perform catheter angiography twice, one week apart. Both angiograms must be negative. Although the cause of the hemorrhage is unknown, it is thought to represent angiographically occult venous bleeding.
Although the clinical presentation of perimesencephalic subarachnoid hemorrhage is similar to aneursymal SAH (thunderclap headache), patients generally do well without residual neurological deficit. Some patients may experience mild to moderate vasospasm.
Reversible cerebral vasoconstruction syndrome (RCVS)
Reversible cerebral vasoconstriction syndrom (RCVS) is a cause of nontraumatic, nonaneurysmal subarachnoid hemorrhage and ischemia. RCVS presents with thunderclap headache and is characterized by prolonged (but reversible) vasoconstriction.
Saccular aneurysm
A saccular (also called berry) aneurysm is a focal outpouching of the arterial wall, most commonly arising at a branch point in the cricle of Willis. The aneurysm points in the direction of blood flow leading into the branch point. Saccular aneurysms are seen almost exclusively in adults, with a slight female predominance. Saccular aneursyms are caused by a combination of hemodynamic stress and acquired degeneration of the vessel wall.
Non-inherited risk factors for the development of saccular aneurysm include hypertension and inflammatory vascular disease such as Takayasu or giant cell arteritis.
Inherited diseases that predispose to anurysm formation include connective tissue diseases such as Marfan and Ehlers-Danlos, polycystic kidney disease, and neurofibromatosis type I.
The aneurysm neck is the opening that connects the aneurysm to the parent vessel and the anuerysm body is the aneurysm sac. The neck:body ratio affects treatment options. Aneurysms with relatively small necks are generally easier to treat endovascularly with coils.
Saccular aneurysms can be classified as small (<1 cm), medium (>1 cm and <2.5 cm), and giant (>2.5 cm). The larger the size, the greater the risk for rupture. Giant aneurysms often present with mass effect, causing cranial nerve palsy.
Fusiform Aneurysm
A fusiform aneurysm is segmental arterial dilation without a defined neck. Fusiform aneurysms are usually due to atherosclerosis, but may arise from chronic dissection.
In contrast to saccular aneurysms, fusiform aneurysms are more difficult to treat. Fusiform aneurysms of the vertebrobasilar system pose particular challenges, as critical perforating vessels may arise directly from the diseased artery.
Mycotic (infectious) aneurysm
Mycotic aneurysms account for only 2-4% of all intracranial aneurysms and are due to septic emboli. Bacterial endocarditis is the most common embolic source.
In contrast to saccular aneurysms, mycotic aneurysms form in the distal areterial circulation, beyond the circle of Willis. Mycotic aneurysms are fragile and have a high risk of rupture.
Oncotic aneurysm
An oncotic aneurysm is an aneurysm is an aneurysm caused by neoplasm.
A benign left atrial myscoma may peripherally embolize and cause a distal oncotic aneurysm.
Traumatic pseudoaneurysm
Aneurysms due to trauma are most commonly pseudoaneurysms, which don’t contain the typical three histologic layers of the vessel wall. Usually the vessel will exhibit abnormal luminal narrowing proximal to the aneurysm. Similar to mycotic aneurysms, traumatic pseudoaneurysms tend to occur distally.
Arteries close to bony structures (such as the basilar and vertebral artery) are prone to dissecting aneurysms.
Venous anatomy
Dural sinuses
The superior sagittal sinus (and its tributaries) drains the motor and sensory strips.
The paired transverse sinuses are usually asymmetric, with the left transverse sinus often hypoplastic.
The sigmoid sinus connects to the jugular bulb.
The torcular Herophili is the confluence of the superior sagittal sinus, the transverse sinus, and the straight sinus. The word torcular is from the Greek word for wine press, and Herophilus was a Greek anatomist. Technically, the term torcular Herophili refers to the depression on the inner table of the skull produced by the confluence of sinuses, but in general use, torcular Herophili refers to the actual confluence of sinuses.
Deep cerebral veins
The deep cerebral veins consist of the paired internal cerebral veins, the basal vein of Rosenthal, and the vein of Galen.
The venous angle (red dot in the diagram above) is the intersection of the septal vein and the thalamostriate veins. The venous angle is the angiographic landmark for the foramen of Monro.
Superficial cerebral veins
The vein of Trolard connects superficial cortical veins to the superior sagittal sinus.
The vein of Labbe drains the temporal convexity into the transverse or sigmoid sinus. Retraction injury to the vein of Labbe during surgery may lead to venous infarction and aphasia.
Venous thrombosis
Thrombosis of a cortical vein or a deep venous sinus is one of the more common causes of stroke in younger patients. Risk factors for venous thrombosis include pregnancy, oral contraceptives, thrombophilia, malignancy, and infection.
A clue to the diagnosis of venous thrombosis on noncontrast CT is increased density eithin the thrombosed sinus or cortical vein (the cord sign). On contrast-enhanced CT, the empty delta sign signifies a filling defect in the superior sagittal sinus.
MR venogram will show how lack of flow in the thrombosed vein or dural venous sinus.
Venous throbosis leads to venous hypertension, which may cause infarction and parenchymal hemorrhage. There are three characteristic patterns of venous infarction, dependent on the location of the thrombosed vein: Superior sagittal sinus thrombosis -> infarction of the parasagittal high convexity cortex. Deep venous system thrombosis -> infarction of the bilateral thalami. Transverse sinus thrombosis -> infarction of the posterior temporal lobe.
CT imaging of intraparenchymal hemorrhage
Noncontrast CT is usually the first imaging study performed in the emergency setting for a patient with a sudden neurologic deficit, headache, seizure, or altered level of consciousness.
CT is highly sensitive for detection of hyperacute/acut intracranial hemorrhage, which appears hyperattenuating relative to brain parenchyma and CSF. Acut hemorrhage may be nearly isoattenuating to water in severe anemia (hemoglobin <8 mg/dL).
MR imaging of hemorrhage
MR imaging of hemorrhage is complex. The characteristics of blood products change on T1- and T2-weighted sequences as the iron in hemoglobin eveloves through phsyiologic stages: Intracellular oxhyemoglobin -> deoxygenation -> intracellular deoxyhemoglobin -> oxidation -> intracellular methemoglobin -> cell lysis -> Extracellular methemoglobin -> chelation -> Hemosiderin and ferritin.
Each stage of this evolution adheres to a reasonably constant time course in the intraxial space and allows the radiologist to “date” the hemorrhage based on the unique characteristics on T1- and T2-weighted images for each stage.
In general, all stages of hemorrhage are isointense or slightly dark on T1-weighted images, except for the methemoglobin stages, which are bright.
Methemoglobin is bright on T1- and T2-weighted images, except for intracellular methemoglobin, which is dark on T2-weighted images.
In general, non hyperacute hemorrhage is dark on T2-weighted images, with the exception of extracellular methemoglobin, which is hyperintense on T2-weighted images. A hyperacute hematoma, containing primarly oxyhemoglobin, is slightly hyperintense on T2-weighted images but features a characteristic dark rim representing deoxygenation at the periphery of the clot.
The inherent slight hyperintensity of oxygenated blood on T2-weighted images becomes apparent in slow flow states, as seen in venous hypertension and moyamoya disease. Slowly flowing blood is not susceptible to the flow-void artifact and the resultant apparently “enhancing” vasculature really represents unmasking of the normal blood signal.
The expected evolution of blood products is highly dependent on macrophage elimination of blood breakdown products. These rules of thumb are not applicable to extra-axial blood and timing is generally not given for extra-axial blood, such as subdural hematoma.
Treatment of hemorrhage
In most cases, imaging is performed to evaluate for a treatable cause of hemorrhage, such as AVM or aneurysm. The mainstay of treatment of intraparenchymal hemorrhage is supportive, including blood pressure control and normalization of any coagulopathy.
Larger hemorrhage can be evacuated surgically if there is significant mass effect or risk of herniation. In particular, a hemorrhage >3 cm in the posterior fossa would generally be treated surgically as there is increased risk of brainstem compression or hydrocephalus from fourth ventricular obstruction.
Specific stages of parenchymal hematoma on MR
Hyperacute hematoma (<6 hours): Intracellular oxyhyemoglobin
- A few hours after red cell extravasation, a hyperacute hematoma is primarily composed of intact red cells containing oxygenated hemoglobin, which is diamagnetic.
- The center of the hematoma will be isointense on T1-weighted images and iso- to slightly hyperintense on T2-weighted images.
- The key finding of a hyperacute hematoma is a peripheral rim of hypointensity on T2-weighted images due to oxygenation of the most peripheral red cells. This peripheral dark rim is most conspicous on GRE sequences.
Acute hematoma (6-72 hours): intracellular deoxyhemoglobin
- After the red cellsdesaturate (lose oxygen), the entire hematoma becomes hypointense on T2-weighted images and iso- to mildy hypointense on T1-weighted images.
Early subacute hematoma (3 days to 1 week): Intracellular methemoglobin
- The subacute phase is characterized by methemoglobin, which is paramagnetic and undergoes proton-electron dipole-dipole interactions (PEDDI) with water. PEDDI shortens T1 to cause hyperintensity on T1-weighted images. Intracellular and extracellular methemoglobin are both hyperintense on T1-weighted images.
- In the early subacture phase, blood remains hypointense on T2-weighted images due to the paramagnetic effects of methemoglobin, which remains trapped in the red cells.
Late subacture hematoma (1 week to months): Extracellular methemoglobin (after RBC lysis)
- The methemoglobin PEDDI effect persists after cell lysis, causing continued hyperintensity on T1-weighted images.
- Paramagnetic effects of methemoglobin lessens. Signal intensity on T2-weighted images inccreases to that of CSF, due to RBC lysis and decreases in prtoein concentration.
- There may be peripheral enhancement of a subacute to chronic infarct.
Chronic sequela of hemorrhage: Hemosiderin and ferritin
- Salvaged iron atoms are deposited into hemosiderin and ferritin, which become permanently trapped in the brain parenchyma after the blood brain barrier is restored.
- Susceptibility effects of the stored iron produce characteristic hypointensity on T2-weighted and GRE images.
- Chronic hemorrhage may have peripheral enhancement.

Hypertensive Intraparenchyma Hemorrhage
Chronic hypertension is the most common cause of spontaneous adult intraparenchymal hemorrhage and is due to the secondary microangiopathic effects of chronic hypertension.
Chronic hypertension causes arteriolar smooth muscle hyperplasia, which eventually leads to smooth muscle death and replacement with collagen. The resultant vascular ectasia predisposes to hemorrhage.
Hypertensive hemorrhage occurs in characteristic locations in the basal ganglia, thalamus, and cerebellum.
In addition to location, imaging (MRI or CT) findings suggestive of a hypertensive bleed include additional stigmata of hypertensive microangiopathy, such as periventricular white matter disease and prior lacunar infarcts.
An additional MR-specific finding suggesting hypertensive hemorrhage is the presence of microhemorrhages on T2* (GRE or SWI) in the basal ganglia or brainstem.
Cerebral amyloid angiopathy (CAA) (Intraparenchymal hemorrage)
Cerebral amyloid angiopathy (CAA) is amyloid accumulated within the walls of small and medium arteris, ultimately causing vessel weakness and increased risk of hemorrhage.
While the spontaneous form of CAA occurs almost exclusively in elderly adults (in which population it is the second most common cause of nontraumatic hemorrhage), a hereditary variant has an earlier age of onset.
In addition to being a risk factor for hemorrhage, CAA can also occlude the lumens of small vessels and contribute to microangiopathy.
The main clinical clue that a hemorrhage is secondary to CAA is that the patient is a normotensive elderly adult.
The primary imaging feature to suggest CAA is the location of hematoma, which is almost always lobar or cortical, usually in the parietal or occipital lobes.
Aneurysmal hemorrhage
As discussed, aneurysmal hemorrhage is by far the most common cause of nontraumatic subarachnoid hemorrhage. If an intraparenchymal hematoma is due to an aneurysm, the hematoma is usually adjacent to the rupture aneurysm dome.
The pattern of subarachnoid blood may help to localize the aneurysm; however, if the patient was found down, then the blood will settle in the dependent portion of the brain, confounding a location.
CT angiography is the study of choice for further evaluation of nontraumatic subarachnoid hemorrhage.
Arteriovenus malformation (AVM)
An arteriovenous malformation is a congenital lesion consisting of abnormal high-flow arteriovenous connections without intervening normal brain.
In case of AVM rupture, the resultant hemtoma is usually parenchymal. In contrast to amyloid angiopathy, a hematoma from a bleeding AVM tends to affect younger patients.
Dural AV fistual (dAVF)
Dural AV fistulas are a group of high-flow vascular malformation characterized by a fistulous connection between a meningeal artery and a venous sinus or cortical vein. Cavernous sinus (cavernous-carotid fistula) and posterior fossa dAVFs are the most common types.
Imaging may show enlarged feeing arteries and enlarged or occluded dural sinuses, or enlarged cortical veins.
Venous thrombosis
Thrombosis of cortical veins or deep venous sinuses leads to venous hypertension, which may cause infarction and parenchymal hemorrhage.
Hemorrhagic neoplasm
Occasionally, the initial presentation of a brain tumor may be acute hemorrhage.
The most common primary brain tumor to cause hemorrhage is glioblastoma.
There are relatively limited number of extracranial primary tumors known to cause hemorrhagic metastases, including: (Choriocarcinoma, Melanoma, Thyroid carcinoma, Renal cell carcinoma, Although breast and lung cancer rarely cause hemorrhage on a per-case basis, they are such common cancers overall that they can always be considered when a hemorrhagic neoplasm is suspected.)
Patients treated with bevacizumab (trade name Avastin, Genetech) may be at increased risk for hemorrhagic metastasis.
Clues to the diagnosis of an underlying tumor causing hemorrhage include more-than-expected edema surrounding a hyperacute hematoma and heterogenous blood product signal, suggesting varying breakdown stages of hemoglobin.
The presence of multiple enhancing masses strongly suggests metastatic disease.
In cases where the diagnosis is unclear, a follow-up MRI should be performed oncethe initial hemorrhage improves. If tumor is present, the MRI may show a delay in the expected evolution of blood products, persistent edema, and enhancement of the underlying tumor.
Cavernous malformation
A cavernous malformation is a vascular hematoma that consists of low-flow endothelial-lined blood vessels without intervening normal brain.
Although non-hemorrhagic cavernomas have a characteristic MRI appearance (with “popcorn-like” lobular mixed/high signal on T1- and T2-weighted images and a dark peripheral hemosiderin rim), once bleeding occurs, the resultant hematoma has nonspecific imaging features. The presence of a developmental venous anomaly adjacent to a hematoma may suggest the diagnosis of a recently hemorrhaged cavernoma.
Angiography plays no role in the diagnosis. Cavernous malformations are angiographically occult.
Hemorrhagic transformation of infarct
In most cases, the clinical or imaging diagnosis of stroke is made before hemorrhagic transformation occurs; however, hemorrhage may occasionally be the presenting feature of an infarct.
More commonly, symptomatic hemorrhage occurs post-infarct in approximately 6-12% of patients receiving thrombolytic therapy.
Noncontrast CT can identify risk factors for hemorrhagic transformation after thrombolytic therapy, including a relatively large region of hypoattenuation and a dense artery sign. Note that per AHA guidelines, neither of these findings is an exclusion criterion for administration of IV tPA.
Vascultitis
Vasculitis affecting the CNS may be primary or secondary to systemic vasculitides.
The most common presentation of vasculitis is cerebral ischemia. Less commonly, vasculitis may present with frank hemorrhage.
Standard MRI imaging shows of vasculitis shows multiple foci of T2 prolongation in the basal ganglia and subcortical white matter.
Noninvasive vascular imaging (CTA or MRA) is relatively insensitive to small vessel involvement, but may show irregularity of involved large or medium vessels.
Angiography is the most sensitive test and shows multifocal areas of stenosis and dilation.
Moyamoya
Moyamoya is a non-atherosclerotic vasculopathy characterized by progressive stenosis of the intracranial internal carotid arteris and their proximal branches, which leads to proliferation of fragile lenticulostriate collateral vessles.
Angiography of the enlarged basal perforating arteries gives a puff of smoke appearance.
The ivy sign on FLAIR MRI represents tubular branching hyperintense structures within the sulci, representing cortical arterial branches that appear hyperintense due to slow collateral flow.
Patients with moyamoya disease are susceptible to aneurysm formation, especially in the posterior circulation. Perfusion studies show decreased flow in the affected vascular regions.
Summary of Hemorrhage Etiology

White Matter Overview
The typical MRI appearance of white matter injury is T2 prolongation of the affected white matter. Less commonly, tumefactive demyelination may be mass-like, enhance, and look very similar to a tumor.
The key imaging finding of demhelinating disease is minimal mass effect relative to the lesion size.
A frequent pattern of white matter disease consisting of scattered foci of T2 prolongation in the subcortical, deep, and periventricular white matter is seen very commonly, especially in older adults. In older patients, a similar pattern can be seen in chronic migraine headaches, as sequelae of prior infectious or inflammatory disease, and with demyelination.
Virchow-Robin spaces are tiny perivascular spaces that follow deep penetrating vessels into the subarachnoid space. Virchow-Robin spaces follow CSF signal on all sequences, including FLAIR. Enlarged Virchow-Robin spaces and a J-shaped sella can be seen in the mucopolysaccharidoses.
Ependymitis granularis represents frontal horn periventricular hyperintensity on T2-weighted images due to interstitial CSF backup. Despite the name (“-itis”), ependymitis granularis is not associated with inflammation.
Multiple Sclerosis
Multiple Sclerosis (MS) is idiopathic inflammatory destruciton of CNS axons in the brain and spinal cord. MS is likely autoimmunein etiology and may be associated with other autoimmune diseases such as Graves disease and myasthenia gravis.
MS is the most common chronic demyelinating disease. It often leads to severe disability.
MS is more common in middle-aged Caucasian females from northern latitudes.
There are two main clincial presentations of multiple sclerosis: 1) Relapsing-remitting (most common): Partial or complete resolution of each acute attack.
2) Progressive: No resolution or incomplete resolution between acute attacks. Primary progressive: Slow onset without discrete exacerbations. Secondary progressive: Similar to relapsing-remitting but with less complete resolution between attacks, leading to progressive disability.
Optic neuritis may represent the first sign of MS. The purpose of a brain MRI after optic neuritis is to look for other lesions, which may be clinically silent.
Histopathologically, destruction of myelin is caused by lymphocytes attacking oligodendrocytes (which makes CNS myelin).
Although MRI imaging is highly sensitive, there are no pathognomonic imaging findings. The McDonald criteria, last revised in 2010, describe strict imaging findings to diagnose MS. The McDonald criteria are most useful for clinically ambiguous cases.
In order to make the diagnosis of MS, there must be lesions separated in space (different areas of the CNS) and in time (new lesions across scans).
Suggestive imaging findings include periventricular ovoid foci of T2 prolongation that “point” towards the ventricles, called Dawson fingers. The corpus callosum is often affected, best seen on sagittal FLAIR.
In general an enhancing lesion is suggestive of active demyelination, as enhancement is thought to be due to inflammatory blood brain barrier breakdown.
Lesions that are dark on T1-weighted images are call “black holes” and are associated with more severe demyelination and axonal loss.
Chronic MS leads to cortical atrophy, thinning of the corpus callosum, and changes in MRI spectroscopy, with decrease in NAA, increase in choline, increase in lipids, and axonal loss.
Tumefactive MS describes the occasionally seen ring enhancement and mass-like appearance of an active MS plaque. In contrast to a brain tumor, the demyelinating lesion will not have any significant mass effect, and the ring of enhancement is usually incomplete.
MS involves the spinal cord in a substantial minority of patients and the spine is routinely evaluated in patients with MS. Spinal MS involvement is usually short-segment and unilateral. Isolated spinal cord involvement is seen in up to 20% of cases of MS.
Concentric (Balo) sclerosis
Concentric (Balo) sclerosis is a very rare variant of MS with pathognomonic alternating concentric bands of normal and abnomral myelin. It is seen more often in younger patients.
Marburg variant (acute multiple sclerosis)
Marburg variant of MS is a fulminant manifestation of MS, leading to death within months.
Devic Disease (neuromyelitis optica)
Devic diseaseis a demyelinating disease, distinct from MS, which involves both the optic nerves and spinal cord. Devic disease confers a worse prognosis compared to MS.
NMO-IgG, an antibody to aquaporin 4, is highly specific for Devic disease. NMO-IgG activates the complement cascade and induces demyelination.
Imaging shows MS-type lesions with involvement of the optic tracts and spinal cord. Brain lesions, if present, tend to be periventricular.
Osmotic Demyelination
Osmotic demylination is caused by a rapid change in extracellular osmolality, typically occuring after aggressive correction of hyponatremia. The quick osmotic gradient change causes endothelial damage, blood brain barrier breakdown, and release of extracellular toxins, which damage myelin.
Patients with poor nutritional status, including alcoholics, chronic lung disease patients, and liver transplant recipient, are the most susceptible to osmotic demyelination.
Osmotic demyelination is typically seen in the pons, but may occur elsewhere in the brainstem and deep gray nuclei. MRI features bilateral central T2 prolongation in the affected region. Signal abnormalities in the thalami and basal ganglia may also be present.
Marchiafava-Bignami
Marchiafava-Bignami is a fulminant demyelinating disease of the corpus callosum seen in male alcoholics.
Wernicke encephalopathy
Wernicke encephalopathy is an acute syndrome of ataxia, confusion, and oculomotor dysfunction, which may be caused either by alcoholism or generalized metabolic disturbances, such as bariatric surgery.
On imaging, there is T2 prolongation and possible enhancement within the mamillary bodies and medial thalamus. The non-alcoholic form may also affect the cortex.
Posterior Reversible Encephalopathy Syndrome
Posterior reversible encephalopathy syndrome (PRES) is a disorder of vasogenic edema with a posterior circulation predominance triggered by failed autoregulation and resultant hyperperfusion, most commonly caused by acute hypertension. In addition to hypertension, PRES is also associated with eclampsia, sepsis, autoimmune disorders, multidrug chemotherapy, and solid or stem cell transplantation.
In contrast to infarction, the edema is vasogenic in etiology, not cytotoxic. Diffusion may be normal, increased, or restricted.
Imaging shows symmetric regions of subcortical white matter abnormality (hypoattenuation on CT and T2 prolongation on MR), especially in the posterior circulation (occipital and parietal lobes are posterior fossa). Mild mass effect and enhancement can be seen.
Cerebral autosomal dominant arteriopathy with subcoritcal infarcts and leukoencephalopathy (CADASIL)
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an inherited disease characterized by recurrent stroke, migraine, subcortical dementia, and pseudobulbar palsy, due to small vessel arteriopathy.
The clincial hallmark of CADASIL is recurrent episodes of stroke or transient ischemic attacks, which are nearly always found to be subcortical in the white matter or basal ganglia on imaging. There is often associated migraine. It may eventually lead to dementia.
MRI shows symmetric foci of T2 prolongation in the subcortical white matter, which may become confluent as the disease progresses. Anterior temporal lobe or paramedian frontal lobe foci of T2 prolongation are highly sensitive and specific for CADASIL, especially with clinical history of migraine. Although the symmetric subcortical pattern is similar to PRES, the distribution in CADASIL is anterior circulation.
Vasculitis
CNS vasculitis is a group of vascular inflammatory disorders that primarily affects the small vessels, in particular the leptomeningeal and small parenchymal vessels.
Etiologies include lupus, polyarteritis nodosa, giant cell arteritis, and Sjogren syndrome.
MRI shows numerous small focal areas of T2 prolongation in subcortical and deep white matter. Although the appearance may be similar to MS, foci of hemorrhage (best seen on GRE or SWI) may be present in vasculitis, which would not be seen in multiple sclerosis.
Vascular imaging (CT angiography and catheter angiography are more sensitive than MR angiography) shows a beaded, irregular apearance to the cerebral vessels.
Microangiopathy
Microangiopathy describes age-related chronic axonal loss, gliosis, and ischemic changes seen in up to 80% of elderly individuals. Microangiopathy never involves the corpus callosum: If involvement of the corpus callosum is present, an alternative diagnosis should be considered, such as multiple sclerosis or neoplasm.
Binswanger disease represents the combination of dementia and severe microangipathy.
Progressive multifocal leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of immunocompromised patients caused by reactivation of JC virus. There is progressive demyelination with lack of inflammatory response.
PML occurs most commonly in AIDS patients (PML is and AIDS defining illness), but can also be seen in patients with malignancy, status post organ transplant, or with autoimmune disorders. Approximately 1 in 1,000 patients with MS treated with natalizumab (Tysabri) may have superimposed PML and it may be difficult to distinguish between MS and PML. Diagnosis is made by PCR for JC virus DNA in CSF.
MR imaging of PML shows asymmetric multifocal white matter lesions that may become confluent. There is rarely mass effect or enhancement. The arcuate (subcortical U) fibers are typically involved. Arcuate fibers are myelinated tracts at the gray-white junction that connect cortex to cortex.
In an AIDS patient, the primary differential for white matter lesions is PML and HIV encephalitis. In contast to PML, HIV encephalitis is usually bilateral (and symmetric), spares the subcortical white matter, and is associated with cerebral atrophy: Diffuse bilateral involvement, sparing of subcortical white matter, and cerebral atrophy -> HIV encephalitis. Asymmetric involvement, involvement of subcortical white matter, and lack of atrophy -> PML.
Subactue sclerosing panencephalitis (SSPE)
Subacute sclerosing panencephalitis (SSPE) is a demyelinating disease causd by reactivation of measles virus, usually after a long latent period.
Imaging shows periventricular white matter lesions, but in distinction to the other white matter entities, SSPE lesions tend to have surrounding edema and mass effect.
Acute disseminated encephalomyelitis (ADEM)
Acute disseminated encephalomyelitis (ADEM) is a monophasic demyelinating disorder seen primarily in children that typically occurs after a viral infection or vaccination.
The majority of patient will make a full recovery, but a minority will have permanent neurologic sequelae.
Imaging findings can be identical to MS, and occasionally cases of presumed ADEM will be reclassified as MS due to polyphasic clinical course. Similar to MS, ADEM may involve the brain, brainstem, or spinal cord.
Optic neuritis and spinal cord involvement can be seen in ADEM, as with MS.
The Hurst variant (acute hemorrhagic leukoencephalitis) is a rapidly fulminant form of ADEM that leads to death within days.
Imaging of the Hurst variant shows multifocal T2 prolongation and associated white matter hemorrhage, which may appear as confluent hematomas.
Radiation Injury
Radiation injury causes small vessel arteritis and secondary ischemia.
The acute phase occurs during radiation therapy, thought to reflect edema due to endothelial injury, and is of little clinical significance.
The early delayed phase occurs between several weeks and up to six months after treatement, and is thought to be due to demyelination. MRI shows diffuse white matter T2 prolongation.
The late delayed phase occurs months to years after radiation. It can present as white matter injury or a focal radiation necrosis. Mass effect, edema, and enhancement are common, and radiation necrosis should be considered in the differential of a ring enhancing mass in a patient with history of prior radiation.
Other complications of brain radiation include the development of meningiomas, capillary telangiectasias, cavernous malformations, and/or moyamoya.
Chemotherapy-related white matter disease
Chemotherapy may cause focal, multifocal, or diffuse white matter disease, especially in combination with radiation treatment.
Pyogenic abscess
A cerebral pyogenic abscess may be due to hematogenous dissemination, direct spread from paranasal sinusitis or mastoiditis, or a complication of bacterial meningitis.
An abscess evolves over four stages (early cerebritis -> late cerebritis -> early abscess -> late abscess) and takes about two weeks to fully develop. In the early stage of infection, there is nonspecific T2 prolongation in the affected region, with heterogenous enhancement.
After the abscess becomes discrete, the classic imaging appearance is a ring-enhancing mass. The rim is hypointense on T2-weighted images. Unlike the rim of a glioma or metastasis, an abscess rim is thin and smooth.
When periventricular in location, an abscess classically tends to feature a thinner capsule oriented towards the ventricles. Disruption of the ventricular margin may herald impending rupture. Rupture may result in ventriculitis, with very high mortality.
A pyogenic brain abscess almost aways demonstrates restricted diffusion, appearing very bright on DWI due to restricted diffusion superimposed on inherent hyperintensity on T2-weighted images.
Tuberculoma
A tuberculoma is a localized tuberculosis granuloma. It is not always possible to differentiate a tuberculoma from a pyogenic abscess.
A tuberculoma tends to have central hypointensity on T2-weighted image (in contrast to a pyogenic abscess, which is hyperintense). A cystic tuberculoma, however, may mimic a pyogenic abscess. Similar to pyogenic abscess, tuberculosis tend to show restricted diffusion.
Lyme disease
Lyme disease, caused by the spirochete Borrelia burgdorferi, can cause white matter disease with a nonspecific imaging appearance of T2 prolongation predominantly in the frontal subcortical white matter. Associated enhancement of multiple cranial nerves or meningeal enhancement may suggest the diagnosis.
Cryptococcus
Cryptococcosis is the most common CNS fungal infection in patients with AIDS and is caused by Cryptococcus neoformans. It is the third most common CNS infection in AIDS overall, after HIV encephalopathy and toxoplasmosis.
Similar to toxoplasmosis, AIDS patietns become susceptible ot cryptococcus with a CD4 count less than 100 cells/uL. The most common clinical presentation is chronic basilar meningitis. The most common imaging finding is hydrocephalus, which is very nonspecific.
Cryptococcus has a predilection for spread to the choroid plexus, producing ring-enhancing granulomas (called cryptoccomas) within the ventricles.
Treatment is with antifungal agents including fluconazole and amphotericin B
Neurocysticercosis
Neurocysticercosis is the most common parasitic CNS infectin of immunocompetent patients. It is caused by the tapeworm Taenia solium and clinically presents with seizures.
Four stages of neurocysticercosis have been described:
1) Viable/vesicular: Imaging shows several CSF-intensity cysts, without enhancement. Many of these cystic lesions may demonstrate an eccentric “dot” representing the scolex.
2) Colloidal: The colloidal stage has the least specific imaging findings, presenting as ring-enhancing lesions. In contrast to a pyogenic abscess, the lesions feature increased diffusivity.
3) Nodular/granular: Edema decreases as the cyst involutes and the cyst wall thickens.
4) Calcified: Imaging shows small parenchymal calcifications (on CT) and small foci of susceptiblity (GRE).
Intraventricular neurocysticercosis can occur in up to 20% of cases, typically in the aqueduct of Sylvius or the fourth ventricle. Hydrocephalus may be the initial presentation, with the obstructing cyst most apparent on FLAIR images because the protein-rich intracystic fluid will not be nulled on FLAIR.
The racemose form of neurocysticercosis is an older term describing a variant without a visible scolex, now thought to represent degeneratin of the scolex.
Treatment is with antiparasitic medications including albendazole, supplemented with steroids for edema and antiseizure medications as needed.
T
Toxoplasmosis
Toxoplasmosis is the most common mass lesion in AIDS patients and is caused by the parasite Toxoplasma gondii. AIDS patients become susceptible to the parasite with a CD4 count less than 100 cells/uL. Toxoplasmosis is the second most common CNS infection in AIDS patients, with HIV encephalitis being the most common.
The typical appearance of toxoplasmosis is single or multiple ring-enhancing lesions in the basal ganglia.
The asymmetric targetsign is not frequently seen but is relatively specific and describes an eccentric nodule of enhancement along the enhancing wall of the toxoplasmosis lesion.
The primary differential consideration of a basal ganglia mass in an immunocompromised patient is CNS lymphoma. In contrast to CNS lymphoma, toxoplasmosis does not demonstrate reduced diffusivity and does not demonstrate increased relative cerebral blood volume on perfusion imaging. Additional studies typically demonstrate toxoplasmosis to be hypometabolic on FDG-PET, and not avid on thallium scintigraphy.
Herpes encephalitis
Herpes encephalitis is a devastating (if untreated), necrotizing encephalitis caused by reactivation of latent HSV-1 within the trigeminal ganglion. Clinical symptoms are nonspecific, with fever and headache often being prominent complaints. Mental status may be altered.
Herpes should be the first consideration in any patient with fever, mesial temporal lobe signal abnormality, and acute altered mental status.
Although lumbar puncture should be obtained before MRI and treatment should never be delayed for MRI, it is important to note that herpes encephalitis may be present when not clinically suspected and PCR of HSV in cerebrospinal fluid is not 100% sensitive.
Herpes encephalitis causes edema, hemorrhage, and necrosis, typicaly in the medial temporal lobes and the inferior frontal lobes.
CT is often normal but can show ill-defined hypoattenuation in the affected regions.
MRI is much more sensitive and typically shows bilateral (but usually asymmetric) T2 prolongation in the medial temporal lobe, insular cortex, cingulate gyrus, and inferior frontal lobe. Once the infection becomes hemorrhagic, MRI will show foci of T1 shortening and gradient susceptibility. The affected areas typically demonstrate reduced diffusion.
Enhancement may develop later in the infection, and is typically gyral in morphology.
In an immunocompromised patient, HSV-6 infection should be considered with the above findings, although enhancement and diffusion abnormalitiesmay be absent.
The differential diagnosis of medial temporal lobe lesions include MCA infarction, infiltrating glioma, limbic encephalitis, and seizure-related changes. Fever is typically absent in infarction and glioma. Herpes encephalitis should be the first consideration in any patient with fever and signal abnormality in the medial temporal lobe.
Treatment is urgent antiviral therapy.
HIV encephalopathy
HIV encephalopathy is the most common CNS infection in AIDS patients. It is a progressive neurodegenerative disease caused by direct infection of CNS lymphocytes and microglial cells (CNS macrophages) by the HIV virus.
On imaging, HIV encephalitis manifests as diffuse cerebral atrophy and symmetric T2 prolongation in the periventricular and deep white matter.
As previously discussed, in contrast to progessive multifocal leukoencephalopathy (PML), HIV encephalitis spares the subcortical U-fibers and tends to be symmetric.
Cytomegalovirus (CMV) encephalitis
Cytomegalovirus (CMV) encephalitis only affects the immunosuppressed, typically when the CD4 count is less than 50 cells/uL.
The most common CNS manifestation of CMV infection is ventriculitis or meningoencephalitis.
The characteristic imaging featrues of CMV ventricultis includesubependymal FLAIR hyperintensity and enhancement throught the ventricular system.
In neonates, CMV is one of the most common TORCH infections and causes atrophy, encephalomalacia, ventricular enlargement, and periventricular calcification.
Creutzfeldt-Jakob disease
Creutzfeldt-Jakob disease (CJD) is a rare neurodegenerative disease caused by a prion.
The typical MRI appearance of CJD is cortical ribboning, which describes ribbonlike FLAIR hyperintensity and restricted diffusion of the cerebral cortex. The basal ganglia and thalami are also involved. There is often sparing of the motor cortex.
The pulvinar sign describes bright DWI and FLAIR signal within the pulvinar nucleus of the thalamus. The hockey stick sign describes bright DWI and FLAIR signal within the dorsomedial thalamus.
Toxic/Metabolic CNS
The classic brain MRI finding in liver disease is hyperintense signal on T1-weighted images in the globus pallidus and substantia nigra, thought to be due to manganese deposition.
Severe hypoglycemia can show bilateral T2 prolongation in the gray matter, including the cerebral cortex, hippocampi, and basal ganglia.
Hypoxic ischemic encephalopathy (HIE) in adults is caused by circulatory or respiratory failure, leading to global hypoxia/anoxia.
Severe HIE typically affects the gray matter, including the cerebral cortex, hippocampi, and basal ganglia. This distribution is similar to that of severe hypoglycemia. Involvement of the basal ganglia portends a worse prognosis.
MR imaging shows FLAIR and/or DWI hyperintensityof the affected regions.
On CT, there is loss of gray-white differentiation, diffuse cerebral hypoattenuation, and sulcal effacement. The white cerebellum sign describes the typical sparing of the cerebellum, which appears relatively hyperattenuating compared to the affected supratentorial brain.
Methanol poisioning can present with optic neuritis as the first symptom.
Hemorrhagic necrosis of the putamen and white matter edema may follow. Carbon monoxide poisoning typically causes symmetric T2 prolongation and restricted diffusion of the globus pallidus.
Neck Anatomy
The retropharyngeal space is a potential space located posterior to the pharynx, separated from the pharynx by the pharyngobasilar fascia. The retropharyngeal space extends from the base of the skull to the upper mediastinum. Directly lateral to the retropharyngeal space are the carotid and parapharyngeal spaces.
The alar fascia is directly posterior to the retropharyngeal space. The alar fascia separates the retropharyngeal space from another postential space, the danger space, which acts as a trapdoor for infection to travel all the way from the neck to the diaphragm.
The preverterbral space (the anterior component of the perivertebral space in hte suprahyoid neck) is located just anterior to the vertebral body an dis bounded anteriorly by the prevertebral fascia.
The sublingual space is a potential space located at the base of the tongue,nestled between the genioglossus and geniohyoid muscles medially and the sling of the mylohyoid muscle inferiorly and laterally.
Directly inferior to the sublingual space is the U-shaped sumbandibular space. The sublingual space is separated from the submandibular space by the mylohyoid muscle anteriorly, although the sublingual space is contiguous with the submandibular space posteriorly.

Ludwig angina
Ludwig angina is cellulitis of the floor of the mouth. It is an infection that can involve the submental, sublingual, and submandibular spaces.
The tongue can rapidly become posterioly displaced, so ensuring airway patency is a clinical priority.
On imaging, there is stranding and swelling at the floor of the mouth.
Retropharyngeal Abscess
Retropharyngeal infection may cause airway compromise.
In children, retropharyngeal infection is most often from spread of an upper respiratory tract infection, such as pharyngitis. Enlargement of retrophayngeal lymph nodes that drain the pharynx may lead to subsequent suppuration and rupture.
In adults, retropharyngeal infection is most often due to penetrating injury, such as fish bone ingestion or instrumentation.
Peritonsillar abscess
Peritonsillar abscess is a complication of peritonsillar lymph node suppuration, causing the characteristic hot-potato voice.
Lemierre syndrome
Lemierre syndrome is venous thrombophlebitis of the tonsillar and peritonsillar veins, often with spread to the internal jugular vein. Immunocompetent adolescents and young adults are typically affected.
The most common infectious agent is the anaerobe Fusobacterium necrophorum, which is part of the normal mouth flora.
Imaging shows enlargement , thrombosis, and mural enhancement of the affected veins.
Metastatic pulmonary abscesses may be present.
Bezold abscess
Bezold abscess is a complication of otomastoiditis where there is necrosis of the mastoid tip and resultant spread of infection into the adjacent soft tissue.
On imaging, there is opacification of the middle ear and mastoid air cells, often with bony erosion of the mastoid.
Ranula
A ranula is a mucous retention cyst that arises from the sublingual gland as a sequela of inflammation. All ranulas arise from the sublingual gland and hence begin in the sublingual space.
A plunging ranula extends from the sublingual space into the submandibular space by protruding posterioly over the free edge of the mylohyoid or by extending directly through a defect in the mylohyoid.
A dermoid cyst may also present below the mandible, but is typically midline, rather than the eccentric location of a plunging ranula.
Dermoid/epidermoid
A dermoid is a teratomatous lesion that contains at least two germ cell layers, while an epidermoid contains only ectoderm.
On CT, both dermoid and epidermoid will appear as a fluid-attenuation lesion, most commonly in the midline of the floor of the mouth. A pathognomonic MRI finding of dermoid is the sack of marbles appearance from floating fat globules. In the absence of this finding, epidermoid and dermoid can beindistinguishable in this location on MRI.
Thyroglossal duct cyst (TDC)
A thyroglossal duct cyst (TDC) is due to persistance of the thyroglossal duct. The thyroglossal duct follows the midline descent of the embryonic thyroid gland from the base of the tongue (foramen cecum) to its normal position in the neck. Most thyroglossal duct cysts present in childhood as an enlarging neck mass that elevates with tongue protrusion.
The majority of TDCs (65%) are infrahyoid, the rest found at the level of the hyoid or above. Most TDCs are midline, but they may occur slightly off midline,especially when infrahyoid.
Thyroid carcinoma (papillary type) is a rare complication seen in 1% of TDCs.
Cystic tongue metastasis
Papillary thryoid cancer and squamous cell carcinoma from the base of the tongue or the tonsils may metastasize to the floor of the mouth region. These metastases tend to be cystic.
First branchial cleft cyst (BCC)
A first branchial cleft cyst (BCC) is rare and is usually located near the parotid or the external auditory canal.
A first BCC may appear as a simple cyst; however, imaging is variable and contents may be heterogenous, especially if superinfected.
Second branchial cleft cyst
A branchial cleft cyst (BCC) is a congenital anomaly arising from the embyrologic branchial apparatus. There are four types of congenital branchial celft cysts, with the second type being the most common type.
A second BCC may occur at any point along the path extending from the palatine tonsil to the supraclavicular region, but most occur near the angle of the mandible.
The classic location of a second BCC is anterior to the sternoclediomastoid muscle, posterior ot the submandibular gland, and closely associated with the carotid bifurcation.
The cyst may become superinfected. With superinfection, the wall may enhance and there may be inflammatory changes within the surrounding soft tissues. Although second BCC as been described as being in the posterior submandibular space, an infected second BCC would not typically be confused with a submandibular abscess. Submandibular abscess is usually due to dental disease and is typically located immediately inferior to the mandible.
Branchial cleft cysts are uncommon in older adults. A cystic metastasis (e.g., from papillary thryoid cancer or base of tongue/tonsillar squamous cell carcinoma) should be considered instead, especially if the cyst is irregular or has a mural nodule.
Submandibular or masticator abscess
A submandibular or masticator abscess may arise from dental disease (most commonly), suppurative adenopathy, or spread of adjacent salivary gland infection.
On imaging, an abscess appears as an irregular, thick-walled, peripherally enhancing fluid collection located inferior to the mandible. Adjacent stranding of the cervical fat is usually present.
In addition to antibiotics, aspiration is usually a key component of treatment.
Lymphatic malformations (neck)
Lymphatic malformations are congenital abnormalities that result when the embyrologic lymphatics fail to connect to developing veins. There are three types of lymphatic malformations, classified by the size of the intra-lesional cystic spaces.
Cystic hygromais the most common type of lymphatic malformation, the majority being present at birth and associated with chromosomal anomalies including Turner and Down syndromes. A cystic hygroma features large lymphatic spaces. The most common location is the posterior triangle of the neck.
Cavernous lymphangioma has smaller lymphatic spaces than a cystic hygroma, while a capillary lymphangioma has the smallest cystic spaces.
Thornwaldt cyst
A Thornwaldt cyst is a notochordal remnant that is usually asmyptomatic, but may be a cause of halitosis. The typical location of a Thornwaldt cyst is in the midline nasopharynx.
Lingual thyroid
Lingual thyroid is ectopic thyroid tissue that has incompletely descended during embyrologic development and remains at the floor of the mouth. Usually, the ectopic gland is the only functioning thyroid tissue, so the neck should be evaluated to confirm lack of normal gland.
Lingual thyoid is much more common in females.
Lingual thyroid is susceptible to standard thyroid pathology, including thyroiditis and cancer.
Larynx anatomy
The layrnx is a fibrocartilaginous tube that extends from the skull base to the esophagus.
The cartilaginous components of the larynx include the epiglottis, thyroid cartiage, cricoid cartilage, and arytenoids. The cricoid cartilage is a complete ring that provides support for the larynx.
The supraglottic larynx extends from the epiglottis to the ventricle. The false vocal cords, the aryepiglottic folds, and the arytenoid cartilages are within the supraglottic larynx.
The glottis includes the true vocal cords and the thyroarytenoid muscle. The medial fibers of the thyroarytenoid muscle comprise the vocalis muscle. The true vocal cords are identified in the axial plane on CT or MRI by identifying the transition of paraglottic fat to muscle (thyroarytenoid muscle) within the wall of the larynx.
The subglottic larynx begins 1 cm inferior to the apex of the laryngeal ventricles and extends to the first tracheal ring.

Vocal cord paralysis
Vocal cord paralysis is most commonly due to iatrogenic trauma from neck surgery but may be secondary to a mass lesion along the course of the vagus or recurrent laryngeal nerves. The most common mass lesion to cause vocal cord paralysis is a mediastinal or thoracic mass; however, enlargement of the left atrium or pulmonary arteries may cause cardiovocal syndrome due to recurrent laryngeal nerve compression.
The CT imaging of vocal cord paralysis shows a thickened, medialized aryepiglottic fold and enlargement of the piriform sinus on the affected side.
The left recurrent laryngeal nerve is the most commonly affected nerve. Imaging for vocal cord paralysis should extend from the skull base to the level of the left pulmonary artery to cover the full course of the vagus and the left recurrent laryngeal nerves. The recurrent laryngeal nerve innervates all laryngeal muscles except the cricothyroid muscle which is innervated by the superior laryngeal nerve.
Laryngocele
A laryngocele is dilation of the laryngeal ventricle, which may be caused by high laryngeal pressures. Trumpet players, glassblowers, and patients with COPD have increased risk of developing a laryngocele. A laryngocele may be filled with air or fluid.
An important differential consideration is ventricular obstruction by neoplasm (most commonly squamous cell carcinoma), which typically causes a fluid filled laryngocele.
Laryngeal Cancer
The vast majority of laryngeal tumors are squamous cellsof the mucosa, which are typically accessible to the otolaryngeologist for direct visualization and biopsy. Less common tumors of the larynx (such as lymphoma and chondrosarcoma) may be submucosal and therefore not visible on laryngoscopic exam.
The role of the radiologist in the workup of laryngeal cancer is not to offer a differential diagnosis, but to determine the extent of disease.
The most important imaging determination is if a tumor is supraglottic, glottic, or subglottic. 30% of tumors are supraglottic. 65% of tumors are glottic. Only 5% of tumors are subglottic. A transglottic tumoris usually defined as a tumor that crosses the laryngeal ventricle to involve both the false and true vocal cords, although the exact definition of a transglottic tumor varies between authors. The presence of a transglottic tumor has important treatment implications.
A small isolated supraglottic or glottic tumor can be treated with laser resection and preservation of laryngeal integrity. In contrast to the more superiorly located tumors, even a small subglottic or transglottic tumor requires a total layngectomy,which is an extremely morbid surgery.
Laryngeal trauma
Blunt trauma to the neck may compress the larynx against the cervical spine. The thyroid cartilage and cricoid cartilage are susceptible to fracture, which may be difficult to detect in young patients with incomplete ossification of these structures.
Laryngeal trauma may also result from intubation, which can cause arytenoid dislocation.
Anatomy of the Paranasal Sinuses
The sinuses are lined with ciliary mucosa that propels secretions through the drainage pathways. Each pathway should be carefuly evaluated on a sinus CT.
The osteiomeatal unit (OMU) is teh common drainage pathway for the maxillary, frontal, and anterior ethmoid sinuses, which all drain into the middle meatus. Several air passages and a single bony structure (the uncinate process) make up the OMU, which is comprised of the maxillary sinus ostium, infundibulum, uncinate process, hiatus semilunaris, ethmoid bulla, and middle meatus. Some authors include the frontal recess as part of the OMU.
Isolated sinus disease of the maxillary sinus is most likely due to obstruction of the maxillary sinus ostium or infundibulum, while sinus disease affecting the maxillary, frontal, and anterior ethmoid sinuses is most likely due to obstruction of the hiatus semilunaris.
The maxillary sinus is the largest sinus and drains via ciliary action through the superiorly located maxillary sinus ostium.
The ethmoid sinus is comprised of multiple small air cells, diveded into anterior and posterior divisions. The basal lamella demarcates the boundary between the anterior and posterior ethmoids. The lateral wall of the ethmoid sinus is the lamina papyracea, a thin bone separating the ethmoid sinus from the orbit.
The anterior ethmoids drain via the frontal recess into the middle meatus (via the OMU). The posterior ethmoids drain via tiny ostia underneath the superior turbinate into the superior meatus.
The frontal sinus is absent at birth and represents an enlarged anterior ethmoid cell. The frontal sinus drains into the ethmoid infundibulum via the frontal recess.
The sphenoid sinus drains into the ethmoid cells via the sphenoethmoidal recess. Subsequently, the posterior ethmoid cells drain into the superior meatus via individual unamed ostia.

Paranasal sinuses turbinates and meatuses
The turbinates are three paired bony protuberances within the nasal cavity.
The superior meatus is adjacent to the superior turbinate. The posterior ethmoids and sphenoid sinus (via the sphenoethmoidal recess) drain into the superior meatus.
The middle meatus is the air channel lateral to the middle turbinate and is the common drainage pathway of the maxillary, frontal, and anterior ethmoid sinuses via the OMU.
The inferior meatus is just inferior to the inferior turbinate and is the drainage pathway of the lacrimal duct.
Postsurgical evaluation of chronic sinusitis
Chronic sinusitis is thought to be caused by obstruction of the normal drainage pathways. The goal of functional endoscopic sinus surgery (FESS) is to relieve the obstruction.
It is essential to report certain anatomic variations that may lead to surgical complications if the surgeon is unaware of them.
A dehiscent lamina papyracea appears on imaging as a medial bulging of the orbit. If the surgeon does not know about this, the orbit may be entered by accident.
Similarly, a dehiscent cribiform plate may produce inferior bulging of the frontal lobe, which can be entered by mistake at endoscopic surgery.
Imaging of the sinuses
CT is the primary modality for imaging the sinuses.
A low mA CT technique has a similar radiation dose to a standard four-view radiographic series, and therefore, there is no role for radiography in the evaluation of sunus disease.
MRI is not typically used in the evaluation of routine sinus disease. The MRI imaging of sinus secretions is complex, with varying signal intensities on T1- and T2-weighted images depending on the chronicity. It is possible for desiccated secretions to show low signal on T2-weighted images that could even be mistaked for normal, while CT would clearly show the fully opacified sinus. MRI does play a limited role in evaluating for suspceted complications of sinusitis, such as osteomyelitis.
Agger nasi cell
The agger nasi (Latin for nasal mound) cell is the most anterior ethmoid air cell. A large agger nasi cell may cause obstruction of the frontal recess.
Haller cell
A Haller cell is an ethmoid cell located inferior to the orbit, which may compromise the maxillary ostium if the Haller cell becomes large or inflamed.
Onodi cell
An Onodi cell is the most posterosuperior ethmoid air cell, which is located directly inferomedial ot the optic nerve. An Onodi cell may be mistaken for the sphenoid sinus endoscopically, potentially placing the optic nerve at risk.
Concha Bullosa
Concha bullosa is formed when the inferior bulbous portion of the middle turbinate is pneumatized. A concha bullosa is usually incidental, but may cause septal deviation and narrowing of the infundibulum when large.
Acute sinusitis
Acute sinusitis is infection or inflammation of the paranasal sinuses and is a clinical diagnosis. Imaging is indicated only if a complication of sinusitis is suspected.
An air-fluid level may represent acute sinusitis, but is nonspecific. Sinus mucosal thickening most commonly represents chronic sinusitis, but can also be seen in acute sinusitis.
Chronic sinusitis
Chronic sinusitis is inflammation of the paranasal sinus mucosa that lasts for at least 12 consecutive weeks. It is an extremely common disease, affecting more than 30 million people annually in the US.
Similar to acute sinusitis, chronic sinusitis is a clinical diagnosis, whith imaging reserved for presurgical planning or to evaluate for suspected complications.
Chronic sinusitis is thought to be caused by obstruction of the normal drainage pathways, and the goal of functional endoscopic sinus surgery (FESS) is to relieve the obstructing lesion.
Orbital complications of sinusitis
Direct spread of infection fromt eh sinuses to the orbits may cause subperiosteal abscess, orbital cellulitis, or opthalmic vein thrombosis.
Intracranial complications of sinusitis
Direct spread of infection into the cranial cavity can cause cavernous sinus thrombosis, meningitis, or abscess. Intracranial abscesses secondary to sinusitis may be epidural, subdural, or intraparenchymal.
Bony complication of sinusitis
Chronic inflammation of the mucoperiosteum may cause periostitis and osteomyelitis.
Osteomyelitis of the frontal bone may cause a subgaleal abscess with associated soft-tissue edema, which is called Pott’s puffy tumor.
Mucous retention cyst
A mucous retention cyst is a common incidental finding representing obstruction of teh small mucosal serous or mucinous glands.
Chronic allergic fungal sinusitis
Chronic allergic fungal sinusitis is a noninvasive disease caused by a hypersensitivity reaction to fungi. Most patients with allergic fungal sinusitis are immunocompetent but have a history of asthma.
On imaging, the affected sinus is expanded and airless, with thin deossified walls. The sinus contents are typically mixed attenuation with heterogenous curvilinear high attenuation.
Acute invasive fungal sinusitis
Acute invasive fungal sinusitis is an aggressive infection that occurs in immunosuppressed patients. Aspergillus and Zygomycetes are the most common organisms. In particular, Aspergillus can cause acute fulminant disease.
The imaging of early invasive fungal sinusitis shows nonspecific sinus mucosal thickening. Later in the disease process there if often local invasion, bony destruction, and intracranial and intraorbital spread.
Unlike chronic allergic fungal sinusitis, invasive fungal sinusitis is not hyperdense on CT.
Antrochoanal Polyp
An antrochoanal polyp is a benign polyp extending from the maxillary sinus into the nasal cavity, with characteristic widening of involved ostium. It may erode bone and extend into the nasopharynx. Complete resection is necessary to prevent recurrence.
Mucocele
A mucocele is an expanded airless sinus that results from obstruction of the sinus ostia.
Mucocele may be secondary to inflammatory sinus disease (most commonly) or tumor.
On imaging, there is thinning of the sinus walls. The contents of the sinus tend to be homogenous on CT and MR; however, the MRI signal intensity is variable depending on the degree of desiccation of the sinus contents. Specifically, with increasing chronicity, signal increases on T1-weighted images and decreases on T2-weighted images. In late disease, there can be markedly reduced signal on T2-weighted images that may actually simulate a normal air-filled sinus, so it’s important to always look at all sequences.
Inverted Papilloma
An inverted papilloma is a benign lobulated epithelial tumor of the sinus mucosa; however, it can be associated with squamous cell carcinoma 10-20% of the time.
The classic imaging finding on an ehnanced study is a cerebriform pattern of enhancement, which describes curvilinear , gyriform ehnancement. In contrast to a mucocele or obstructed secretions, the entire solid tumor will enhance. The tumor tends to remodel bone.
Treatment is surgical resection and recurrences occur in approximately 15%.
Anatomy of the salivary glands
The parotid, submandibular, and sublingual glands are the major salivary glands. There are also numerous unamed minor salivary glands in the head, neck, and trachea.
The parotid glands are the largest salivary glands. Each parotid gland is divided into superficial and deep lobes, although there is no fascial demarcation between these lobes. Surgeons use the facial nerve as the demarcation between the superficial and deep lobes. Since the facial nerve is not normally visible on imaging, radiologists use the retromandibular vein as the demarcation.
The main parotid duct is known as Stensen’s duct.
During embryological development, the parotid gland is the last major salivary gland to become encapsulated, and is therefore the only salivary gland that contains intrinsic lymphoid tissue.
The facial nerve exits the skull at the stylomastoid foramen and subsequently passes anterior to the posterior belly of the digastric and lateral to the styloid process before entering the parotid gland.
The paired submandibular glands sit partly in the floor of the mouth, and partly in the neck. Each gland wraps around the free posterior margin of the mylohyoid muscle, which separates the floor of the mouth from the neck.
The sublingual glands are the smallest major salivary glands. Each gland sits medial to the mandible at the anterior aspect of the floor of the mouth.
There are numerous tiny minor salivary glands spread throughout the mucosa of the paranasal sinuses, oral and nasal cavities, nasopharynx, and trachea.
Normally, minor salivary glands are not seen on imaging; however, occasionally a neoplasm may arise from one of these normally invisible glands.
The most common minor salivary gland tumor is the bening pleomorphic adenoma; however, a tumor of minor salivary gland origin is much more likely to be malignant than a tumor arising from the submandibular or parotid glands.
Pleomorphic adenoma (parotid)
Pleomorphic adenoma is by far the most common parotid tumor, accouting for 80% of all parotid tumros. The tumor typically presents as a small firm mass in a middle-aged patient. There is a slight female predominance.
Although pleomorphic adenoma is benign, complete surgical resection is the standard treatment. Left unexcised, the tumors can continue to grow, and there is an increasing risk for malignant transformation to carcinoma ex pleomorphic adenoma. Additionally, it is not possible to distinguish between bening pleomorphic adenoma and malignant mucoepidermoid carcinoma by imaging alone.
During surgery, the capsule of the tumor must be preserved to prevent diffuse tumor seeding.
CT is insensitive for detecting a small parotid tumor, so MRI is preferred. The T1 and T2 characteristics of a pleomorphic adenoma are similar to water. Unlike a simple cyst, however, enhancement is typical for pleomorphic adenoma.
Warthin tumor (parotid)
Warthin tumor is the second most common benign parotid tumor, accounting for 10% of all parotid tumors. Up to 15% are bilateral and the typical patient is an elderly male. Smoking is a risk factor. Unlike pleomorphic adenoma, there is no risk of malignant transformation.
Warthin tumor generally appears as a cystic neoplasm. Unlike pleomorphic adenoma, warthin tumor does not ehance.
Mucoepidermoid carcinoma (parotid)
Mucoepidermoid carcinoma is relatively uncommon (accouting for approximately 5% of all parotid tumors), but it is the most common primary parotid malignancy.
Low-grade mucoepidermoid carcinoma typically appears as an enhancing mass that is hyperintense on T2-weighted images. This appearance is indistinguishable from benign pleomorphic adenoma on MRI.
Treatment is surgical resection.
Adenoid cystic carcinoma (parotid)
Adenoid cystic carcinoma is the most common submandibular and sublingual gland malignancy and the second most common parotid gland malignancy.
Adenoid cystic carcinoma has a tendency to spread along the nerves (perineural spread) and often presents with cranial nerve palsy or paresthesia.
Adenoid cystic carcinoma has a very high risk of local recurrence.
Metastatic disease is frequently present (in up to 50%). Even with a large metastatic burden, however, patients may survive for several years.
The key imaging feature suggestive of adenoid cystic carcinoma is an enhancing mass with perineural spread.
Carcinoma ex pleomorphic adnoma (parotid)
A benign pleomorphic adenoma has a 1.5% risk of malignant degeneration during the first 5 years. That risk increases to 9.5% in tumors present for 15 years.
Carcinoma ex pleomorphic adenoma tends to affect elderly patients, with the classic clinical presentation of rapid enlargement of an existing mass.
In contrast to benign pleomorphic adenoma, malignant carcinoma ex pleomorphic adenoma is hypointense on both T1- and T2-weighted images.
Squamous cell carcinoma (parotid)
Primary squamous cell carcinoma of the parotid gland is very rare, as there are normally no squamous epithelial cells within the parotid. Chronic inflammation however, can induce squamous metaplasia.
Imaging demonstrates an aggressive, large mass, often with nodal metastases.
Sialolithiasis and obstructive sialadenitis
Sialolithiasis (stone disease of the salivary ducts) can cause obstruction of the duct and resultant inflammation of the salivary gland, referred to as obstructive sialadenitis. The vast majority of salivary calculi (80-90%) occur in the mucus-producing submandibular gland, due to its relatively viscous and alkaline secretions. Additionally, the submandibular duct has an uphill course, which predisposes to stasis.
Sarcoidosis (parotid)
Involvement of the parotid gland is seen in up to 30% of patients with sarcoidosis, typically presenting as bilateral painless swelling.
Uveoparotid fever, which presents with bilateral uveitis, parotid enlargement, and facial nerve palsy, is considered pathognomonoic for sarcoidosis.
Gallium-67 scintigraphy produces the classic panda sign from increased uptake by the lacrimal and parotid glands.
Sjogren syndrome (parotid)
Sjogren syndrome is an autoimmune disorder that affects the major and minor salivary glands as well as the lacrimal glands. When secondary to a systemic connective tissue disorder such as rheumatoid arthritis, the disease is referred to as secondary Sjogren syndrome. When only the salivary and lacrimal glands are affected, the disease is called primary Sjogren syndrome.
Sjogren syndrome typically affects middle-aged females.
On imaging, there is atrophy and fatty replacement of the salivary glandular tissue, with multiple nodules, abnormal enhancement, numerous small cystic foci, and punctate calcification.
The risk of parotid and head and neck lymphoma is markedly increased in patients with Sjogren syndrome. Any new dominant parotid mass in the settin gof Sjogren syndrome should raise concern for lymphoma.
HIV lymphoepithelial lesions
Patients with HIV frequently have parotid manifestations of lymphoid dysfuction, including multiple bilateral lymphoepithelial cysts and solid masses.
The parotid glands are the only salivary glands containing intrinsic lymphoid tissue. Therefore, only the parotid glands are affected in patients with lymphoepithelial lesions.
Anterior skull base and pterygopalatine fossa anatomy
The pterygopalatine fossa is the bridge between the face and the brain, and is an important potential pathway for the spread of tumor or infection.
The pterygopalatine fossa sits directly posterior to the maxillary sinus and inferior to the inferior orbital fissure. It can be thought of as a three-dimensional box with each of the six sides leading to important structures in the face.
The pterygopalatine fossa should contain symmetric fat and soft tissue. Asymmetry in the pterygopalatine fossa should raise concern for a mass, such as lymphoma or perineural spread of a salivary tumor (e.g., adenoid cystic carcinoma).
The contents of the pterygopalatine fossa are the pterygopalatine ganglion and branches of the internal maxillary artery.
The pterygomaxillary fissure is the lateral exit of the pterygopalatine fossa, which leads into the masticator space.
The sphenopalatine foramen is the medial exit of the pterygopalatine fossa. It leads into the nasopharynx via the superior meatus. The nasal margin of the sphenopalatine foramen is thought to be the site of origin of juvenile nasopharyngeal angiofibroma.
Foramen rotundum is the posterior opening to the middle cranial fossa. Cranial nerve V2 travels through foramen rotundum.
The inferior orbial fissure is the “roof” of the pterygopalatine fossa and opens anteriorly into the orbit. The infraorbital nerve and artery travel through the inferior orbital fissure.
The vidian canal is located directly below foramen rotundum. It contains the vidian nerve and vidian artery, also known as the nerve and artery of the pterygoid canal.
The pterygopalatine canal is the “floor” of the pterygopalatine fossa, which leads to the oral cavity via the greater and lesser palatine foramina. The pterygopalatien canal transmits the descending palatine nerve and artery.
The anterior skull base divides the anterior cranial fossa superiorly fromt eh paranasl sinuses and orbits inferiorly.
The cribiform plate of the ethmoid bone is the roof of the nasal cavity.

Juvenile nasopharyngeal angiofibroma (JNA)
Juvenile nasopharngeal angiofibroma (JNA) is a benign, highly vascular tumor seen in adolescent males. The most common clinical presentation is nasal obstruction and epistaxis.
Despite the lack of metastatic behavior, JNA is very locally aggressive and insinuates through adjacent skull base foramina. Tumors frequently recur if incompletely resected.
JNA arises from within the nasal aspectof the sphenopalatine foramen, which is the medial boundary of the pterygopalatine fossa.
On imaging, JNA enhances very avidly and is centered in the nasopharynx. As the mass continues to grow, extension into the pterygopalatine fossa or the orbits is commonly seen.
The three classic findings of JNA include: 1. Nasopharyngeal mass. 2. Expansion of the pterygopalatine fossa. 3. Anterior bowing or displacement of the psoterior maxillary sinus wall.
Pre-operative embolization is often performed to reduce the vascularity of the lesion prior to resection.
Olfactory groove meningioma
Meningioma, a common benign neoplasm arising from archonoid villi rests, is the most common intracranial lesion to affect the anterior skull base.
The imaging appearance of olfactory groove meningiomas is similar to that of meningiomas elsewhere, featuring avid enhancement and an ehancing dural tail. There is often reactive bone sclerosis.
Overview of skull base tumors
It is usually not possible to arrive at a single diagnosis when faced with a neoplasm involving the anterior skull base. However, the radiologist plays an important role to assist the surgeon in determining resectability and optimal surgical approach.
Important findings to describe include the presence of bony destruction, invasion into the brain parenchyma, and extension into the orbit and cavernous sinus.
Invasion into the brain may manifest soley as vasogenic edema, even without abnormal enhancement.
Orbital invasion most commonly occurs through the lamina papyracea, which is the weak medial orbital wall.
Esthesioneuroblastoma
Esthesioneuroblastoma, also known as an olfactory neuroblastoma, is a malignant neural crest tumor that arises form specialized olfactory epithelium. The histology is similar to other neural crest tumros, such as small cell lung carcinoma and neuroblastoma.
Esthesioneuroblastoma affects patients in a bimodal age distribution, with peak incidence in the teenage years and middle age.
On imaging, the tumor appears as an aggressive mass that is slightly hyperattenuating on CT and intermediate intensity on both T1- and T2-weighted images due to high cellularity. Calcification is often present.
A classic finding, thought by some authors to be pathognomonic of esthesioneurblastoma, is the presence of peripheral tumor cysts that occur at the margins of the intracranial portion of the mass.
Squamous cell carcinoma (skull base)
Sqamous cell carcinoma (SCC) is by far the most common malignancy of the paranasal sinuses and the nose. The maxillary antrum is the most common primary site within the paranasal sinuses.
On imaging, SCC of the skull base appears as an aggressivve, intensely enhancing mass with bony destruction. Enhancement is the key differentiating feature from benign inflammatory processes such as sinonasal polyposis or mucocele.
Adenoid cystic carcinoma (skull base)
Adenoid cystic carcinoma may arise from minor salivary glands in the sinonasal cavity.
Although local lymphatic spread is rare, distant metastases are seen commonly.
Adenoid cystic carcinoma demonstrates water signal on T1- and T2-weighted images, but unlike a cyst, enhancement is characteristic.
Adenoid cystic carcinoma arising in the region of the anterior skull base has a high rate of trigeminal nerve perineural spread. Post-contrast evaluation of the cranial nerves offers the highest sensitivity to evaluate for subtle perineural spread.
Rhabdomyosarcoma
Rhabdomyosarcoma is the most common head and neck tumor in children. It is discussed in the pediatric imaging section.
Overview of the temporal bone
The temporal bone can be anatomically and functionally divided into the external, middle, and inner ear. Although some pathologies can overlap, each division tends to be susceptible to unique pathologies.
This anatomic and functional division parallels the pathway of sound waves into the brain. Sound enters the head at the external ear, is amplified by the ossicles in the middle ear, and is finally converted into electrical impulses in the inner ear.
An additional functiona of the inner ear (not drawn above) is the balance and position sense provided by the vestibule and the semicircular canals. Together, the cochlea, vestibule, and semicircular canals make up the labyrinth.
The external auditory canal (EAC) is a fibrocartilaginous tube that sound passes through before reaching the middle ear.

Congenital EAC stenosis, hypoplasia, and atresia
Congenital malformations of the external ear, including stenosis, hypoplasia, and complete atresia, are relatively common. The EAC and inner ear have different embryologic origins, so it is uncommon for both an EAC anomaly and an inner ear abnormality to be present.
The malleus and incus are 1st branchial cleft structures and are usually abnormal in EAC atresia.
Acute external otitis
Acute external otitis, otherwise known as swimmer’s ear, is a bacterial infection of the external ear seen in areas of high humidity.
EAC exostosis (surfer’s ear)
EAC exostosis, also known as surfer’s ear, is a bony exostosis of the EAC seen in thos who swim/surf in cold waters.
Necrotizing external otitis (malignant otitis externa)
Previously known as malignant otitis externa, necrotizing external otitis is a severe complication of external otitis seen in elderly diabetic or immunocompromised patients. The most common pathogens are Pseudomonas aeruginosa in diabetics and Aspergillus fumigatus in the immunocompromised.
The infection may be fast-spreading and aggressive, with potential involvement of the middle or inner ear structures, or skull base extension.
On imaging, there is extensive ehancement centered around the external ear, with associated bony erosion.
Keratosis obturans
Keratosis obturans is characterized by keratin plugs within an enlarged EAC, and is typically seen in young patients with sinusitis and bronchiectasis. It is usually bilateral.
Cholesteatoma
Acquired cholesteatomas occur more commonly in the middle ear, but should be considered in the differential of an EAC soft tissue mass, especially in the presence of bony erosion.
EAC malignancy
The most common malignancy of the external auditory canal is squamous cell carcinoma. Risk factors are controversial but may include sun exposure or chronic malignancy.
Anatomy of the middle ear and adjacent facial nerve
The middle ear begins at teh tympanic membrane and includes the ossicles (malleus, incus, and stapes) and the tensor tympani and stapedius muscles.
The handle of the malleus is attached to the tympanic membrane. Sound waves vibrate the tympanic membrane, which transmits mechanical energy directly to the malleus, and then through the malleoincudal and incudostapedial articulations to the oval window. The footplate of the stapes articulates directly with the oval window.
The oval window is the interface between the air-filled middle ear and the fluid-filled inner ear.
Glomus typanicum
A glomus tympanicum is an extra-adrenal pheochromocytoma (paraganglioma) isolated to the middle ear. Glomus typanicum is the most common kprimary middle ear tumor and typically presents with pulsatile tinnitus or conductive hearing loss.
On otoscopic evaluation, a vascular red mass will be present behind the tympanic membrane.
Facial nerve schwannoma
Within the temporal bone, schwannomas of the facial nerve (CN VII) tend to be slow growing and most commonly involve the geniculate ganglion, followed by the labyrinthine and tympanic segments. The segments of the facial nerve in the temporal bone are: Labyrinthine segment (courses from the internal auditory canal to the geniculate ganglion, which is located superior to the cochlea. Greater superficial petrosal nerve innervates salivation). Tympanic (horizontal) segment. (Facial nerve courses under the lateral semicircular canal). Mastoid (descending) segment. (Facial nerve courses inferiorly, then exits the temporal bone at the stylomastoid foramen. Nerve to stapedius. Chorda tympani (taste to anterior 2/3 of tongue).
The majority of patients with facial nerve schwannoma present with facial nerve palsy; however, up to 30% without facial nerve symptoms. Larger facial nerve schwannomas is enlargement of the bony canal through which the involved portion of the facial nerve passes. There is typically no calcification. The tumor usually demonstrates brisk contrast enhancement on MRI.
Cholesterol granuloma (cholesterol cyst)
A cholesterol granuloma, otherwise known as a cholesterol cyst, is the most common benign petrous apex lesion, but may also occur in the middle ear. It is caused by a giant cell reaction to cholesterol crystals thought to be initially incited by an obstructed air cell.
On otoscopic examination, a blue mass is present behind the tympanic membrane.
Cholesterol granuloma and cholesteatoma are oten considered together in the differential diagnosis of a soft tissue middle ear mass with bony erosion. In contrast to cholesterol granuloma, a cholesteatoma will show restricted diffusion and will appear as a white mass on otoscopic examintion of the tympanic membrane. Cholesterol granuloma is typically hyperintense on T1-weighted images.
Cholesteatoma
A cholesteatoma is a non-neoplastic lesion of the temporal bone which has been described as “skin in the wrong place”. The name cholesteatoma is deceptive since the lesion does not contain fat and is not neoplastic. An intracranial, extra-axial “cholesteatoma” is an epidermoid cyst.
Cholesteatomas can occur in children or adults. Cholesteatomas of the middle ear are more common in younger individuals, while cholesteatomas of the EAC are more common in middle-aged and older adults.
A middle ear cholesteatoma is almost always acquired (accounting for 98% of all middle-ear cholesteatomas), but rarely may be congenital. There is no histopathologic difference between the congenital and acquired forms. a congenital cholestatoma can occur anywhere within the temporal bone, while acquired cholesteatomas occur in the middle ear.
The etiology of cholesteatomas is controversial. Acquired cholesteatoma may represent sequela of tympanic membrane perforation, inflammation, or traumatic implantation of epidermal elements. Congenital cholesteatoma may represent persistent fetal epithelial squamous cell nests.
Clinically, congenital cholesteatomas are usually asymptomatic, but may be detectable as a white mass behind the tympanic membrane. If the cholesteatoma grows large, symptoms may mimic acquired cholsteatoma with recurrent ear disease, eustachian tube obstruction, chornic ear discharge, and mixed sensorineural and conductive hearing loss.
In the evaluation of a suspected cholsteatoma, it is important to describe potential involvement of three key landmarks: The lateral semicircular canal, the tegmen tympani (the bony roof separating the mastoids from teh brain), and the facial nerve.
CT is very sensitive for the detection of even a small cholesteatoma, but is nonspecific and cannot differentiate between cholesteatoma and other soft tissue masses such as cholesterol granuloma or neoplasm. The typical CT appearance of cholesteatoma is a well-circumscribed soft-tissue mass with adjacent bony erosion, blunting of the scutum, and erosion of the ossicles.
The most specific MRI sequences to evaluate for cholesteatoma are post-contrast and diffusion-weighted imaging. Similar to intracranial epidermoids, cholesteatomas are hyperintense on diffusion-weighted (DWI) images and do not enhance. The DWI hyperintensity represents a combination of T2 shine through and restricted diffusion, thought to be due to the viscous contents of the cholesteatoma. Cholesteatomas are intermediate to slightly hyperintense on T2-weighted images and of variable signal intensity on T1-weighted iamges.
Anatomy of the Inner ear
The inner ear consists of the otic capsule. The otic capsule is the bony superstructure containing the fluid-filled spaces of the cochlea, vestibule, and semicircular canals. The otic capsule is the densest bone in the body.
The cochlea allows us to hear. It is a two-and-a-half turn spiral containing numerous neuroepithelial hair cells of the organ of Corti, which send electrical impulses tot he spiral ganglia of the cochlea in response to mechanical bending. Hydraulic pressure created in the perilymph by the vibrations of the stapes against the oval window ascends to the apex of the cochlea by the scala vestibuli. Pressure waves descend back to the basal turn through the scala tympani. The vibrations from teh scala tympani are transmitted through the round window, where the energy is dissipated.
The vestibule is invovled with balance. The semicircular canals are three arcs oriented in orthogonal planes that allow perception of movement in three-dimensional space.
The vestibular aqueduct connects to the crus common (the common channel of the superior and posterior semicircular canals) to the subarachnoid space at the internal auditory canal, and is thought to be involved in pressure equalization between the CSF and the inner ear.
Cochlear dysplasia (Mondini deformity)
The most common form of congenital cochlear dysplasia is characterized by incomplete development of the normal two and a half turns of the cochlea, resulting in confluence of the apical and middle turns and preservation of a distinct basilar turn.
The correct nomenclature for this anomaly is incomplete partition type II, although it is commonly referred to as the Mondini deformity. The use of the eponym Mondine to describe cohclear dysplasia is controversial and probably best avoided, as there is confusion amongst radiologists regarding the exact malformation originally described.
There is a strong association between cochlear dysplasia and enlarged vestibular aqueduct.
Michel dysplasia
Michel dysplasia is a very rare complete lack of development of the entire inner ear.
Enlarged vestibular aqueduct syndrome
Although the vestibular aqueduct does not have a direct function in the physiology of hearing, there is a spectrum of congenital hearing loss associated with an enlarged vestibular aqueduct. Clinically, enlarged vestibular aqueduct syndrome may lead to progressive hearing loss while playing contact sports.
As an internal reference, the vestibular aqueduct should not be larger than the posterior semicricular canal, which is often seen at the same level in the axial plane.
Otospongiosis (otosclerosis)
Otospongiosis, otherwise known as otosclerosis, is a primary bone dysplasia of the otic capsule characterized by replacement of normal endochondral bone by irregular spongy bone. Otospongiosis occurs most commonly in young and middle-aged womem and is bilateral 85% of the time.
The two main types of otospongiosis are fenestral and retrofenestral types.
The fenestral type of otospongiosis is more common, occurs at the fissula ante fenestrum (located directly anterior to the oval window), and usually affects the oval window.
The retrofenestral (cochlear) type is thought to represent a mroe severe form with involvement of the otic capsule in addition to the lateral wall fo the labyrinth. The differential diagnosis of cochlear demineralization includes retrofenestral otospongiosis, osteogenesis imperfecta in a child, fibrous dysplasia in a young adult, and Paget disease in an older adult.
CT is the best imaging modality for evaluation of otospongiosis, which shows increased lucency of the affected bone. Fidnings may be extremely subtle without detailed knowledge of the normal appearance of the otic capsule.
Labyrinthitis
Labyrinthitis is inflammation of the inner ear, which may be infectious or autoimmune.
Labyrinthitis has been divided into three stages of chronicity based on the imaging (primarily MR) characteristics.
Acute labyrinthitis is the earliest stage, presenting as pus in the inner ear. The only MRI signal abnormality of acute labyrinthitis is enhancement of the affected inner ear structures. The main differential consideration of acute labyrinthitis is a chochlear or intralabyrinthine schwannoma. A schwannoma tends to be focal and discrete, while labyrinthitis may produce diffuse enhancement.
Fibrous labyrinthitis represents the replacement of endolymph and perilymph with fibrous strands that caused decreased signal intensity on T2-weighted images. There may be mild (but decreased) residual post-contrast enhancement of the affected structures.
Labyrinthtitis ossificans is the final stage of the disease. Calcified debris replaces the normal endolymph and perilymph. CT is the best way to image labyrinthitis ossificans, as the calcification causes decreased signal intensity on T2-weighted MRI and lack of enhancement.
Temporal bone fractures
Temporal bone fractures have been historically classified by their orientation with respect to the axis of the temporal bone. A newer classification that has been most predictive of clinical outcome divides temporal bone fractures into otic capsule-violating versus otic capsule-sparing, although the longitudinal and transverse terms remain in common use.
Longitudinal temporal bone fracture
A longitudinal temporal bone fracture is aligned with the long axis of the petrous portion of the temporal bone and is by far the most common type of temporal bone fracture. Longitudinal temporal bone fractures are likely to involve the ossicles and result in conductive hearing loss. 20% of longitudinal fractures are associated with facial nerve injury.
If the tympanic membrane is disrupted, there is increased risk for subsequent development of an acquired cholesteatoma.
Transverse temporal bone fracture
A transverse temporal bone fracture is perpendicular to the long axis of the petrous portion of the temporal bone. Transverse fractures are more likely to involve the bony labyrinth and result in sensorineural hearing loss. 50% of transverse fractures result in facial nerve injury.
Anatomy of the petrous apex
The petrous apex is the most medial portion of the temporal bone.
The petrous apex is a bridge between the suprahyoid neck inferiorly and the intracranial compartment above, and is adjacent to several important structures such as Dorello’s canal, Meckel’s cave, and the petrous portion of the internal carotid artery. Cranial nerve VI passes through Dorello’s canal. Meckel’s cave is the site of the trigeminal ganglion.
Normally, the petrous apex is composed of bone marrow and dense bone. The petrous apex is pneumatized in approximately 10% of the population, which increases the risk for development of a cholesterol cyst or apical petrositis.
Cholesterol Cyst (Cholesterol granuloma)
A cholesterol cyst is a foreign body giant cell reaction to cholesterol crystals. It is thought to be initially instigated as a reaction to an obstructed air cell and therefore occurs more commonly in a penumatized petrous apex.
A cholesterol cyst is the most common primary petrous apex lesion but may also occur in the mastoid portion of the temporal bone or the middle ear.
MR imaging of a cholesterol cyst shows an expansile mass with internal hemorrhage and fluid that does not suppress on fat suppresion, unlike fatty marrow.
Apical petrositis (petrous apicitis)
A relatively rare complication of infectious otomastoiditis, apical petrositis is also known as petrous apicitis and results when infection extends medially into a pneumatized petrous apex.
In the early stages of apical petrositis, there is opacification of the petrous apex air cells with progressive bony demineraliztion and resorption, resulting in localized osteomyelitis of the skull base.
The classic Gradenigo triad is not commonly seen, but is comprised of otomastoiditis, facial pain due to trigeminal neuropathy at Meckel’s cave, and lateral rectus palsy from 6th cranial nerve palsy at Dorello’s canal.
Vascular complications of apical petrositis include internal carotid arteritis and dural venous thrombosis.
Congenital cholesteatoma
While acquired cholesteatomas are located within the middle ear, congenital cholesteatoma can be located anywhere in the temporal bone, including the petrous apex.
Mri imaging will typically show restricted diffusion.
Schwannoma
A petrous apex schwannoma may originate from cranial nerves V, VII, or VIII. Imaging shows a circumscribed, smoothly expansile, enhancing mass that may cause bony remodeling. Large tumors may become cystic and contain fluid levels.
Langerhans cell histiocytosis (eosinophilic granuloma)
Langerhans cell histiocytosis (LCH) is a neoplastic proliferation of eosinophils and Langerhans cells. The temporal bone is the most common site of skull base inolvement.
CT sshows a well-circumscribed destructive lesion, usually with nonsclerotic margins.
MRI shows the LCH lesions as soft-tissue masses surrounded by bone marrow and soft tissue edema. Marked enhancement is characteristic.
Chondrosarcoma
Chondrosarcoma is a malignant neoplasm that characteristically arises in the midline from the clivus or slightly off-midline from the petroclival synchondrosis. CT often shows a tumor with a ring-and-arc chondroid matrix, although internal matrix is not always seen. MRI shows a lobular, cauliflower-shaped, hyperintense mass on T2-weighted images.
Chordoma
Chordoma (subsequently discussed in the section on clival lesions) most commonly arises fromt eh clivus but may expand laterally to secondarily invovle the petrous apex. In some circumstances it can be difficult to distinguish between chordoma and chondrosarcoma.
Differential diagnosis of a petrous apex lesion

Clivus
The normal clivus in an adult should demonstrate fatty marrow signal on sagittal T1-weighted MRI. In children, the clival marrow may be low in signal due to persistent hematopoietic marrow, depending on age. While diffuse marrow replacement may represent a systemic process, focal replacement of normal marrow should raise concern for neoplastic disease.
Chordoma
Chordoma is a locally invasive tumor arising int he midline from notochord remnants. Approximately 50% arise near the sacrum, 35% near the clivus, and the remainder in the vertebral column.
Chordomas do not have internal matrix but calcification within the lesion may represent residual fragments of eroded bone.
MR imaging of chordoma is similar to chondrosarcoma, appearing as a hyperintense mass on T2-weighted iamges with bony destruction.
Chondrosarcoma
Chondrosarcoma may arise either centrally from the clivus or para-sagittally from the petrous apex.
Metastasis
Breast cancer has a propensity to metastasize to the clivus
Paragangliomas (Glomus tumors)
Paragangliomas, otherwise known as glomus tumors, are neoplasms of paraganglionic tissue that arise from sympathetic glomus bodies. These neoplasms are histologically identical to extra-adrenal pheochromocytoma. Paragangliomas occur in a few predictable locations in the head and neck. Five named glomus tumors have been described, classified by the typical locations at which they arise. Most glomus tumors are benign, but they can be locally aggressive. Less than 5% undergo malignant degeneration.
Paragangliomas are associated with multiple endocrine neoplasia type 1 and neurofibromatosis type 1, where they are often multiple.
Paragangliomas are highly vascular and enhance avidly.
The classic MRI imaging pattern is the salt and pepper appearance due to intra-tumoral flow voids.
Conventional angiography may be performed to provide a vascular road map and to perform pre-surgical embolization.
Glomus jugulare
Glomus jugulare is the most common primary neoplasm of the jugular foramen. The typical patient is a woman in late middle age presenting with pulsatile tinnitus and conductive hearing loss.
The moth-eaten bony destruction typical of paraganglioma is centered around the jugular foramen. A schwannoma or meningioma may also occur near the jugular foramen; however, schwannoma typically features smooth bony remodeling and meningioma typically causes hyperostosis.
Glomus typanicum
Glomus tympanicum is a paraganglioma isolated to the middle ear.
Otoscopic evaluation shows a vascular, red, retro-typanic mass. The differential diagnosis of a vascular, red, retro-tympanic mass includes:
Glomus tympanicum.
Aberrant carotid artery.
Tympanic membrane hemangioma.
Glomus jugulotympanicum
Glomus jugulotypanicum is a glomus jugulare that has spread into the middle ear.
Carotid body tumor (glomus caroticum)
Glomus caroticum, much more commonly referred to as a carotid body tumor, is a paraganglioma of the carotid body.
It characteristically splays the internal and external carotid arteries.
Glomus vagale
Glomus vagale is a paragangioma of the vagus nerve, typically occurring at the same level of the neck as the carotid body tumor.
Glomus vagale displaces the carotid artery (both internal and external branches) medially and anteriorly. In contrast, a carotid body tumor splays the intenral and external carotids.
A schwannoma of the vagus nerve may occur in the same location and will also displace the carotid artery medially and anteriorly. A schwannoma is generally relatively less vascular. Schwannoma may enhance avidly, but would not show the flow voids typical of a glomus vagale.
Bony orbits and foramina anatomy
The orbit is a quadrilateral, pyramidal, bony cavity made of five bones: the frontal, ethmoid, nasal, zygomatic, and the maxilla. Several of these bones are very thin and several contain pneumatized sinuses. The bony surfaces are covered with a tough periosteum, which often maintains the integrity of the orbit even in the presence of a bony fracture.
The following structures pass through the optic foramen: Optic nerve, Opthalmic artery.
All of the cranial nerves (CN) of the cavernous sinus except V2 enter the superior orbital fissure. V2 exites the cavernous sinus via the foramen rotundum and subsequently enters the orbit via the inferior orbital fissure. The following structures pass through the superior orbital fissure: CN III (oculomotor nerve) innervates the superior, medial, and inferior rectus, and the inferior oblique. CN IV (trochlear nerve) innervates the superior oblique (nmenominc: SO4). CN V1 (opthalmic division of the trigeminal nerve) provides senosry innervation to the upper face. CN VI (abducens nerve) innervates the lateral rectus (mnemonic LR6). Superior opthalmic vein, a valveless vein that can be a potential route of infection into the brain.
The following structures pass through the inferior orbital fissure:
CN V2 (maxillary division of trigeminal nerve) provides sensory innervation to the inferior eyelid, upper lip, and nose.
Infraorbital artery.
Compartments of the orbits
The orbit can be conceptually and anatomically divided into five compartments to aid in the differential diagnosis and classification of orbital pathology, although several typesof masses (e.g. orbital hemangioma and lymphoma) may not be confined to these boundaries.
Division into preseptal and postseptal compartments is most useful for the evaluation of orbital infection. The orbital septum is thin connective tissue at the orbital margin that extends into the tarsus of the eyelid. The preseptal orbit is an anterior superficial potential space that does not contain any orbital structures.
The postseptal orbit, which is essentially the entire orbit, can be further divided into four compartments: The extraconal, conal, and intraconal compartments, and the globe.
The extraconal compartment contains the lacrimal gland, fat, and bony orbit.
The conal compartment contains the extraocular muscles. All of the muscles except for the inferior oblique muscles arise from a common fibrous ring called the annulus of Zinn, located at the apex of the orbit at the optic foramen and medial superior orbital fissure. The inferior oblique arises from the medial orbital floor.
The intraconal ocmpartment is comprised of the structures contained within the cone of the extraocular muscles, including the optic nerve-sheath complex consists of the optic nerve, surrounding CSF, and leptomeningeal and dural coverings.
The globe is considered a distinct compartment.
Overview of orbital infection
Direct spread of infection from the paranasal sinuses is the most common etiology of orbital infection.
Other etiologies of orbital infection are trauma, foreign body, and odontogenic infection.
Orbital infection represents a progressive spectrum, listed below from least to most severe.
Preseptal infection
Infection of the superficial preseptal space presents with swelling and erythema of the eyelids.
Orbital cellulits/phlegmon
Orbita cellulits or phlegmon is infection within the orbit that is not yet organized or peripherally ring enhancing.
Any postseptal infection, including orbital cellulits, may spread intracranially via the valveless opthalmic veins and predispose to intracranial infection.
Subperiosteal abscess
Subperiosteal abscess is an organized infection underneath the orbital periosteum, which clinically presents with exopthalmos and visual impairment.
Subperiosteal abscess is a surgical emergency due to the risk of permanent blindness from elevated intraocular pressure.
Treatment with with intravenous antibiotics and surgical drainage.
Orbital abscess
Orbital abscess is a more severe form of infection than subperiosteal abscess, clinically presenting with opthalmoplegia in addition to the symptoms of subperiosteal abscess.
Cavernous sinus thrombosis
Cavernous sinus thrombosis is a severe sequea of orbital infection. It may present with multiple cranial nerve palsies in addition to symptosm of orbital abscess.
Hemangioma
There are two types of orbital hemangioma. Cavernous hemangioma is seen in adults and is the most common adult orbital mass, typically presenting with progressive proptosis. In contrast, orbital capillary hemangioma is rare and is seen only in the first year of life.
Orbital cavernous hemangioma (adult hemangioma) is usually intraconal but may be extraconal. It is composed of vascular channels filled with slowly moving blood. CT shows an ovoid, enhancing intraconal or extraconal mass. Bony remodeling may be present. MRI shows a well-defined mass that is isointense on T1-weighted images and hyperintense on T2-weighted images, demonstrating early patchy enhancement that progressively fills in.
Capillary hemangioma (pediatric hemangioma) is analogous to and often present in conjunction with associated skin lesions such as port-wine stain and strawberry hemangioma. Like these lesions, capillary hemangioma typically enlarges over the first few months of life before spontaneously involuting.
Orbital lymphoma
Orbital lymphoma is usually associated with systemic disease. The lacrimal gland, located in the superolateral quadrant of the orbit,is the most common site of orbital lymphoma. When lymphoma is centered on the lacrimal gland, mass effect on the globe leads to the classic clinical presentation fo painless downward proptosis.
Orbital lymphoma is a hypercellular tumor that is hyperdense on CT and hypointense on both T1- and T2-weighted imaging.
Orbital lymphoma and orbital pseudotumor both tend to mold to the globe, although orbital pseudotumor typically presents with pain. Other orbital masses may deform the globe.
Lymphangioma
Lymphangioma is a benign hamartomatous lesion seen in the pediatric population. The preferred nomenclature for lymphangioma is low flow lymphatic malformation.
Lymphangiomas most commonly involve the extraconal compartment but may be found anywhere in the orbit.
On imaging, lymphangioma appears as a multilocular cystic mass, often with complex internal contents and fluid levels from prior hemorrhage. Slight peripheral and septal enhancement is often seen.
Schwannoma/neurofibroma
Scwannoma and neurofibroma are peripheral nerve sheath tumors with indistinguishable imaging appearances when they occur in the orbit. Schwannoma is more common.
The most commonly affected nerves are the sensory branches of V1, resulting in a characteristic location in the superior orbit. Involvement of other nerves, however, can produce a schannoma anywhere in the orbit. Regardless of which nerve is involved, the location tends to be eccentric within the orbit.
Orbital nerve-sheath tumors tend to be hypointense on T1-weighted images, hyperintense on T2-weighted images, and demonstrate enhancement.
Metastasis (orbits)
In the adult, breast, lung, thyroid, renal cell, and melanoma are known to metastasize to the orbit. A classic imaging finding is the appearance of metastatic scirrhous breast cancer, which can prevent exopthalmos or even cause frank enopthalmos due to fibrosis.
A special consideration in children is metastatic neuroblastoma, which classically causes an aggressive sunburst-type periosteal reaction within the involved bone.
Lacrimal gland lesion
The lacrimal gland is composed of both epithelial salivary tissue and lymphoid tissue, and is therefore subject to diseases of these two tissue types.
Epithelial tumors of the lacrimal gland are approximately 50% benign (e.g. pleomorphic adenoma) and 50% malignant (e.g. adenoid cystic and mucoepidermoid carcinoma).
Diseases of lymphoid tissue, wuch as sarcoidosis and lymphoma, may also affect the lacrimal glands. The lacrimal gland is the most common site of orbital lymphoma.
The lacrimal gland may also become enlarged in orbital pseudotumor.
Thyroid opthalmopathy (thyroid eye disease)
Thyroid ophthalmopathy (thyroid eye disease) is orbital inflammation associated with thyroid disease. It is mediated by lymphocytes that produce hyaluronic acid. Ultimately, this process leads to fibrosis of the extraocular muscles. Although thyroid opthalmopathy is most commonly associated with Graves disease, it can occur in any thyroid disorder, even prior to the development of lab abnormalities.
Initially, there is an increase in intra-orbital fat, which may demonstrate stranding due to inflammatory change. Subsequently, the extraocular muscles become enlarged.
The inferior rectus muscle is most commonly affected first. The mnemonic I’M SLow helps remember the ordre of involvement of the extraocular muscles: Inferior rectus -> medial rectus -> superior rectus -> lateral rectus
Isolated lateral rectus enlargement is very uncommon in thyroid ophthalmopathy and should prompt a search for an alternative diagnosis, such as orbital pseudotumor.
In contrast to pseudotumor, tyroid ophthalmopathy tends to be bilateral and spares the muscle tendons.
Steroids are temporarily helpful, but 20% of patients will ultimately require surgery to pervent optic nerve compression at the orbital apex.
Orbital pseudotumor
Orbital pseudotumor is idiopathic orbital inflammation mediated by an infiltrate of lymphocytes, plasma cells, and macrophages. Clinically, it presents with painful proptosis (in contrast to lymphoma, which is usually painless). Orbital pseudotumor is a diagnosis of exclusion that can only be made after other causes of orbital inflammation (e.g., Wegener granulomatosis, sarcoidosis, and others) have been ruled out.
The lacrimal gland is the most commonly involved orbital structure. Several other structures in the orbit may be affected, including the anterior structures (producing uveitis) and the extraocular muscles.
Imaging findings of orbital pseudotumor include stranding within the orbit fat, increased orbital soft tissue, and enlargement of the extraocular muscles.
Treatment is with steroids.
Tolosa-Hunt syndrome is the same pathologic process as orbital pseudotumor, but involving the cavernous sinus.
Optic nerve glioma
Optic nerve glioma is the most common tumor to arise from the optic nerve-sheath complex.
Optic nerve glioma can affect children or adults, with differing prognosis. The tumor of childhood is typically a low-grade astrocytoma with an indolent course. Childhood tumors are associated with neurofibromatosis type 1 and are often bilateral. In contrast, optic nerve glioma of adulthood is usually an anaplastic astrocytoma or glioblastoma multiforme, with a poor prognosis.
The MRI appearance of the low-grade childhood tumoris variable. Often there is fusiform enlargement of the optic nerve and variable enhancement. Like low-grade pilocytic astrocytomas of the posterior fossa, the tumor may have a cyst and nodule appearance.
The aggressive adult optic glioma typically appears as an enhancing mass, which often extends intracranially to involve the optic chiasm. The tumor may involve any part of the optic tract, including the optic radiations.
Optic nerve meningioma
Optic nerve meningioma is the second most common optic nerve-sheath tumor, arising from arachnoid cells within the leptomeninges surrounding the optic nerve.
The typical patient is a middle-aged female with slowly progressive visual impairment, classically with preservation of the central visual field.
On imaging, the nerve-sheath tends to be circumferentially thickened, with uniform contrast enhancement. The enhancing peripheral tumor and the nonenhancing, central optic nerve produce the tram-track sign on the axial image.
Optic neuritis
Optic neuritis is non-neoplastic inflammation of the optic nerve. It is typically painful and presents with painful subacute vision loss and reduced color perception.
Optic neuritis is most commonly due to multiple sclerosis, but may result from other etiologies including viral infection, sarcoidosis, vasculitis, and toxin exposure. Optic neuritis associated with spinal demyelination (in the absence of brain lesions) is Devic syndrome, previously discussed in the section on white matter imaging.
Acutely, there is optic nerv enlargement and T2 prolongation of the optic nerve. Enhancement suggests active disease. Chronically, the optic nerve atrophies.
Imaging of the brain and spine should be performed to evaluate for intracranial plaques: More than 75% of patients with optic neuritis will have a brain white matter lesion.
Globe lesions
Lesions of the globe are most commonly seen in children
Retinoblastoma
Retinoblastoma is the most common primary malignant tumor of the globe.
Retinoblastoma is almost always seen in children under 5 years old and typically presents with leukocoria (white pupillary reflex).
Sporadic cases are often unilateral, but familial cases (associated with p53tumor suppressor mutation) are bilateral.
Classically, retinoblastoma appears as a hyperattenuating, enhancing retinal mass with calcification, in a normal-sized globe.
“Trilateral” retinoblastoma describes bilateral retinoblastoma with pineal-gland pineoblastoma. “Quadrilateral” retinoblastoma is bilateral retinoblastoma, pineoblastoma, and suprasellar retinoblastoma.
Coat disease
Coat disease is a vascular disease of the retina that affects boys and features lipoproteinaceous subretinal exudates that lead to retinal detachment. Patients with Coat disease tend to be slightly older than those affected by retinoblastoma.
The globe is normal in size but features subretinal soft tissue that does not enhance.
Retinopathy of prematurity (ROP)
Retinopathy of prematurity (ROP) is seen only in premature infants and is due to prolonged oxygen therapy. Findings include abnormal vascular development, hemorrhage, and retinal detachment.
Both eyes tend to be affected equally.
There is bilateral micropthalmia (small globes), with increased attenuation of the globe due to prior hemorrhage.
Though intraocular calcifications may be present (similar to retinoblastoma) ROP is distinguished from retinoblastoma by micropthalmia.
The end-stage is pthisis bulbi, which is a shrunken, nonfunctioning globe.
Persistent hyperplastic primary vitreous (PHPV)
Persistent hyperplastic primary vitreous (PHPV) is persistent embryonic vasculature within the vitreous that leads to loss of vision from hemorrhage, cataracts, and retinal detachment. Affected infants are typically full term.
The eye is small (micropthalmia) with increased attenuation of the vitreous.
PHPV is distinguished from retinoblastoma and ROP by the absence of calcifications.
Coloboma
A coloboma is caused by incomplete fusion of the embryonic intraocular fissure, which can result in an elongated or malformed globe.
Coloboma is associated with numerous syndromes including trisomies 13 and 18, and the CHARGE and VATER associations.
Imaging shows a cone-shaped or notch-shaped deformity. Optic nerve colobomas can have outpouchings posteriorly, while iris colobomas are anterior. Colobomas that involve the uvea tend to have micropthalmos and a cyst.
Sphenoid wing dysplasia
Sphenoid wing dysplasia is seen in neurofibromatosis type 1.
Transmission of CSF pulsation through the sphenoid wing defect may classically produce pulsatile exopthalmos.
Septio-optic dysplasia
Septo-optic dysplasia is characterized by optic nerve hypoplasia and agenesis of the septum pellucidum.
It is often associated with schizencephaly, a full-thickness cleft in the cerebral hemisphere that creates a communication between the ventricles and the extra-axial subarachnoid space.
Overview of the head and neck spaces
The complex layers of cervical fascia produce several fascially bounded spaces in the suprahyoid neck. Knowledge of these spaces is useful in creating differential diagnoses for neck masses, as specific lesions tend to arise in characteristic locations.
Pharynx
The pharynx is a muscular tube extending from skull base to the thoracic inlet. It is the anatomic center of the head and neck, around which the other suprahyoid spaces wrap.
The pharynx is divided into five distinct anatomic regions:
Nasopharynx: Top of pharynx behind the nasal cavity.
Oropharynx: From the level of the palate to the hyoid bone, behind the oral cavity. The posterior 1/3 of the tongue is part of the oropharynx.
Oral cavity: The space defined by the anterior 2/3 of the tongue, bounded superiorly by the palate and inferiorly by the floor of the mouth.
Hypopharynx: From the hyoid bone to the esophagus (posterior).
Larynx: From the hyoid bone to the trachea (anterior).
Masticator space
The masticator space is located directly anterior to the parotid and contains the muscles of mastication, the mandible, and cranial nerve V3. Cranial nerve V3 (mandibular division) exits the skull through foramen ovale and innervates the muscles of mastication. If there is a lesion in the masticator space, it is important to assess for perineural spread along V3.
Differential diagonsis of a masticator space lesion:
Odontogenic disease - Dental disease is the most common masticator space pathology, which can lead to abscess.
Mandibular lesion - Osteosarcoma and metastasis are the two most common malignant mandibular lesions.
Rhabdomyosarcoma - Rhabdomyosarcoma is the most common head and neck tumor of childhood.
Anatomy of the carotid space
The carotid space, or post-styloid parapharyngeal space, is an incomplete fascial ring surrounding the carotid artery and jugular vein. The carotid space extends from the skull base to the aortic arch. There is some controversy regarding the terminology of the carotid space, as some authors prefer the term “post-styoid parapharyngeal space” versus “carotid space”. Thought the former is more anatomically accurate because the fascial boundary of this space is incomplete, the latter is simpler and perhaps more helpful to generate a differential diagnosis of a mass.
The contents of the carotid space include the carotid artery, carotid body, jugular vein, and several cranial nerves. The vagus nerve (cranial nerve X) is the only cranial nerve that remains within the entire way into the thorax. In contrast, cranial nerves IX, XI, and XII pass transiently throught the carotid space. Though there are lymph nodes surrounding the carotid space, there are no lymph nodes contained within it.
The pattern of displacement of vascular structures in the carotid space is the key to generating a differential diagnosis for a carotid space mass.
Differntial diagnosis of a carotid space mass
Paraganglioma - A paraganglioma is a benign, highly vascular neoplasm of neural crest cells, featuring intense enhancement and a characteristic salt-and-pepper appearance on MRI due to intra-tumoral flow voids.
Paraganglioma of the carotid body (carotid body tumor) splays the external and internal carotid arteries at the carotid bifurcation.
Paraganglioma of the vagal nerve (glomus vagale) displaces the internal and external carotid arteries anteromedially.
Schwannoma - Similar to glomus vagale, schwannoma (also most commonly of the vagus nerve) also displaces the carotids anteromedially. Schwannoma, however, is not nearly as vascular as paraganglioma and usually does not enhance as homogenously.
Neurofibroma - Neurofibroma are almost always associated with neurofibromatosis type 1.
Neurofibroma and schwannoma are indistinguishable by MRI.
Parapharyngeal space
The parapharyngeal space (PPS) is a triangular fat-filled space with no significant contents aside from occasional ectopic minor salivary gland tissue. The parapharyngeal space is relatively conspicous on both MRI and CT due to its fat content.
The direction of displacement of the parapharyngeal space by a mass lesion in an adjacent compartment is predictable and helpful in determining from which compartment a given mass originates.
Masticator space lesions (e.g., masticator abscess) displace the PPS posteromedially.
Parotid lesions (e.g., pleomorphic adenoma) displace the PPS anteromedially.
Carotid space lesions (e.g., paraganglioma) displace the PPS anteriorly.
Perivertebral space
The perivertebral space is formed by the deep later of the deep layer of deep cervical fascia, which wraps entirely around the prevertebral and paraspinal muscles. The prevertebral space is the anterior component of the perivertebral space. The perivertebral space is in the suprahyoid neck; the paravertebral space in the anaologus region in the thoracolumbar spine.
Contents include vertebral arteries, paraspinal muscles, spinal column, and exiting nerves.
Overview of cervical lymph nodes
The cervical lymph nodes are divided into seven levels as recommended by the AJCC (American Joint Committee on Cancer), with consistent agreement between surgeons, oncologists, and radiologists. The lymph node levels are not defined by fascial planes, but instead are defined by anatomic landmarks and organized by patterns of lymphatic spread.
In an adult, 90% of head and neck cancers are squamous cell carcinoma: The role of the radiologist is not to provide a differential, but to stage the disease. The degree of lymph node involvement has a substantial prognostic value. For instance, a single metastatic lymph node decreases survival from a squamous cell carcinoma by 50% over an equivalent period.

Level I
Level I lymph nodes include submental and submandibular nodes, which are superior to the hyoid bone and inferior to the mandible and mylohyoid.
IA nodes are submental, lying between the medial margins of the anterior bellies of the digastrics.
IB nodes are submandibular, lateral to the medial margin of the anterior belly of the digastric and extending to the posterior margin of the submandibular gland.
Level II
Level II lymph nodes are upper internal jugular nodes, extending from the skull base to the inferior margin of the hyoid bone.
IIA nodes are anterior to the posterior margin of the internal jugular vein (IJV).
IIB nodes are posterior to the IJV but anterior to the posterior margin of the sternocleidomastoid muscle.
Level III
Level III lymph nodes are middle jugular nodes, extending craniocaudally from the inferior aspect of the hyoid to the inferior aspect of the cricoid cartilage. The posterior edge of the sternocleidomastoid is the shared posterior margin for both level III and level IIB nodes.
Level V
Level V lymph nodes are posterior cervical nodes.
VA nodes are superior from the skull base to the inferior cricoid cartilage.
VB nodes are inferior, extending from the inferior cricoid cartilage to the clavicle.
Level VI
Level VI nodes are pretracheal nodes, which are often simply called “pretracheal”. They are located anteromedially in the lower neck and bounded laterally by the carotid sheaths. Level VI extends craniocaudally from the inferior aspect of the hyoid bone to the top of the manubrium.
Level VII
Level VII nodes are superior mediastinal nodes, which are also commonly described by their location. They are inferior to level VI and medial to the carotid sheaths, extending craniocaudaly from the superior aspect of the manubrium to the brachiocephalic vein.
Spine Tumors
The first step in evaluation of any spinal lesion is to determine its compartment of origin. Each compartment has its own differential considerations.
The three compartments are intramedullary (deep to the pia, usually within the substance of the cord itself), intradural-extramedullary (within the dura, but outside the pia), and extradural (external to the dura).

Astroctyoma (spine)
Astrocytoma is the most common intramedullary tumor in children. Most spinal astrocytomas are low grade. Spinal glioblastoma multiforme has been described but occurs rarely. Astrocytoma usually extends over several vertebral levels and causes fusiform dilation of the spinal cord.
Cystic components are seen in approximately 1/3 and a syrinx may be present. Tumors almost always enhance despite being low grade. In contrast to ependymoma, hemorrhage is rare.
Astrocytoma cannot be reliably differentiated from ependymoma based on imaging features. Hemorrhage is mroe commonly seen with ependymoma and may occasionally be a helpful discriminating feature.
Intramedullary lesions
Intramedullary lesions are located deep to the pia, typically within the substance of the cord itself. By definition, all intramedullary lesions are also intradural.
Astrocytoma and ependymoma together make up 95% of intramedullary tumors. These two tumors have overlapping imaging features.
Astrocytomas are more common in children and ependymomas are more common in adults.
Ependymoma (spine)
Ependymoma is the most common intramedullary tumor in adults, arising from ependymal cells that line the central spinal canal. Ependymoma is associated with neurofibromatosis type 2, especially when seen in children.
Ependymoma is often hemorrhage, leading to heterogenous MRI appearance. Peripheral hemosiderin deposition causes a dark rim on T2-weighted images. Most ependymomas enhance. Classically, there is extensive formation of both tumoral cysts and nontumoral polar cysts. Tumoral cysts are surrounded by enhancement and must be resected with the mass. A classic radiographicfinding of ependymoma is scalloping of the vertebral bodies; however, that finding is associated with advanced disease and is rarely seen today.
Hemangioblastoma
Hemangioblastoma is a rare intramedullary tumor (distant third most common behind astrocytoma) associated with von Hippel-Lindau (VHL). One third of hemangioblastomas are associated with this syndrome.
Characteristic imaging features are marked enhancement, cyst formation, and numerous flow voids. Up to 15% have both intramedullary and intradural-extramedullary components.
Demyelinating lesion (spine)
An active multiple sclerosis (MS) lesion may enhance and mimic a spinal tumor, but MS is often distinguishable from a neoplasm by the absence of cord expansion (analogous to the absence of significant mass effect that is typical of intracranial tumefactive MS).
Overview of intradural-extramedullary lesions
Intradural-extramedullary lesions are located within the dura but outside the spinal cord. Most of the time, intradural-extramedullary lesions are located in the subarachnoid space. A classic imaging appearance (not always seen) is a cleft of CSF between the lesion and the cord.
Nerve sheat tumors (neurofibromas and schwannomas) and meningiomas together make up about 90% of intradural-extramedullary tumors.
Nerve sheath tumor (schwannoma, neurofibroma) spine
Benign nerve-sheath tumors are the most common intradural-extramedullary tumors. Fifteen percent are both intradural-extramedullary and extradural (called “dumbell” tumors) and 15% may be completely extradural.
Neurofibroma and schwannoma cannot be reliably differentiated on imaging. Schwannoma is more common, occurs in older patients, and is encapsulated. Schwannoma can be treated by a nerve-sparin approach, which is accomplished by “shelling out” the tumor.
Neurofibroma is associated with neurofibromatosis type 1, occurs in younder patients (early adulthood), and lacks a capsule. The complete circumference of the nerve is involved and treatment therefore necessitates nerve resection.
Both neurofibroma and schwannoma can cause neural foraminal enlargement.
Both neurofibroma and schannoma can cause neural foraminal enlargement.
The target sign, which is central hypointensity on T2-weighted images surrounded by a hyperintense periphery, is seen in both conditions and suggests benignity.
Meningioma (spine)
Meningioma is a benign neopasm arising from arachnoid cap cells. It is most commonly seen in older women.
A classic imaging finding is a broad dural base. Meningiomas often calcify. Typical locations are anterior to the cord in the cervical spine and posterior to the cord in the thoracic spine.
Dermoid cyst (spine)
Dermoid cyst is a congenital lesion that usually presents in childhood.
Dermoid contains macroscopic fat, which is hyperintense on T1-weighted images.
Rare dermoid rupture may cause potentially fatal chemical meningitis.
Epidermoid cyst (spine)
Epidermoid cyst is a rare acquired spinal tumor. It is thought to be caused by implantation of skin elements in the subarachnoid space during neonatal spinal puncture.
Spinal epidermoids are usually intradural-extramedullary but may rarely be intramedullary.
Most commonly, an epidermoid cyst appears similar to a simple cyst (hypointense on T1-weighted images and hyperintense on T2-weighted images). Faint peripheral rim enhancement can be seen. Occasionally, the cyst contents may be proteinaceous, causing T1 shortening.
As with other epidermoids, diffusivity is restricted, in contrast to an arachnoid cyst.
Myxopapillary ependymoma
Myxopapillary ependymoma is an ependymoma variant that occurs exclusively within the conus medullaris or the filum terminale. It is the most common tumor oft he conus and filum and arises from ependymal cells in the filum.
The tumor is characterized by slow growth, classically leading to vertebral scalloping and spinal canal enlargement.
Myxopapillary ependymoma is a highly vascular, hemorrhagic tumor, featuring a xomplex, lobulated MRI appearance with internal hemorrhage. Peripheral hemosiderin is often present, producing hypointensity on T2-weighted images and blooming on GRE.
Arachnoiditis
Arachnoidits is inflammation of the arachnoid surrouding the nerve roots, which produces a fibrinous exudate and secondary dural adhesions. In the past, tuberculosis and syphilis were common causes of arachnoiditis. Today, lumbar spine surgery is a far more common cause. Arachnoiditis may be a cause of persistent back pain after lumbar surgery.
The primary imaging features of arachnoiditis is diplacement of the nerve roots. Three patterns have been described:
Group 1: Central conglomerations of nerve roots within the thecal sac.
Group 2: Peripheral clumping of nerve roots, causing the empty thecal sac sign.
Group 3: Obliteration of the subarachnoid space. Imaging shows increased softtissue within the thecal sac. This is the most severe form of arachnoiditis.
Overview of extradural lesions
Extradural lesions are located external tothe dura. The epidural space is extradural.
Degenerative lesions, such as osteophytes and herniated discs, are the most common extradural lesions.
Other important extradural lesions include epidural metastases and infection (discitis/osteomyelitis).
Primary extradural tumors are rare and usually bony in origin.
Degenerative disease (spine)
Degenerative lesions are the most common extradural lesions, but are usually not difficult to diagnose. Degenerative spine disease is discussed in the next section.
Vertebral body/epidural metastasis
Metastatic disease is the most common adult extradural malignancy. Breast, lung, and prostate are the most common primary tumors to metastasize to the spinal column.
T1-weighted images are essential for evaluation of vertebral body marrow replacement. Marrow should always be more hyperintense on T1-weighted iamges than the intervertebral discs.
Focal decrease in intensity on T1-weighted images is concerning for metastasis.
Diffuse decrease in intensity on T1-weighted images is abnormal but nonspecific. The differential for diffusely decreased T1 marrow signal includes leukemia, lymphoma, myelofibrosis, HIV, and idiopathic causes.
Hemangioma
Vertebral body hemangioma is a common, usually incidentally found, benign lesion composed of endothelium-lined vascular structures.
Destruction of some of the bony trabeculae and thickening of the remaining trabeculae produce the characteristic striated corduroy appearance on plain radiographs and sagittal/coronal CT. The stippled appearance on axial CT is produced by thickened trabeculae seen on end.
Hemangioma is typically hyperintense on both T1- and T2-weighted images.
Although hemangioma is benign, an aggressive variant is occasionally seen in the thoracic spine. The aggressive variant may have an epidural soft tissue component that has the potential to cause cord compression. Although the asymptomatic variant has no sex predilection, the aggressive form of hemangioma is more common in females.
Primary osseous vertebral body tumors of middle-aged and older adults
Chordoma is a malignant tumor of older adults that arises from a notochord remnant. Chordoma occurs most commonly in the sacrococcygeal region and second most commonly in the clivus. Up to 15% may occur in the vertebral bodies (most commonly cervical). Chordoma appears as a destructive, hyperintense mass on T2-weighted images. It avidly enhances.
Plasmacytomas is a lytic, expansile bony lesion of late adulthood that is thought to be a precursor of multiple myeloma. A plasmacytoma is a solitary lesion. The presence of multipe plasmacytomas implies the diangosis of multiple myeloma. Development of fulminant multiple myeloma, or even abnormal serum/urin protein electophoresis, may not occur for many years after diangosis of a plasmacytoma.
Chrondrosarcoma is a low-grade malignancy that appears as a hyperintense mass on T2-weighted images, similar to chordoma. Chonroid rings-and-arcs calcification may be seen.
Primary osseous vertebral body tumors of adolescents and young adults
Aneurysmal bone cyst (ABC) is a benign, progressively destructive lesion that occurs in adolescents. Fluid levels are typically seen on MRI, though this finding is nonspecific.
Chondroblastoma is a benign but aggressive neoplasm that rarely may occur in the vertebral column (vertebral body and posterior elements). Secondary ABC may be present.
Osteoid osteoma is a benign, sclerotic lesion affecting the vertebral posterior elements in teenagers and young adults, which a classic clincal history of nocturnal pain relieved by NSAIDs. Acentral radiolucent nidus consists of vascular fibrous connective tissue.
Osteosarcoma is a malignant tumor that typically produces osteiod matrix.
Epidural lipomatosis
Spinal epidural lipomatosis is a rare overgrowth of fat in the extradural space, which may cause compressi on of neural elements. In severe cases, epidural lipomatosis may cause cauda equina syndrome.
Epidural lipomatosis may be caused by exogenous steroid administration (systemic administration much more commonly than epidural injection) or Cushing disease/syndrome. Epidural lipomatosis is a complication of morbid obesity.
Surgery is the preferred treatment if a neurological deficit is present.
Back pain
Back pain is the second most common reason for physician visits in the US (an upper respiratory complaint is the most common reason).
Trauma or age-related degeneratie changes can cause spinal stenosis, neural foraminal stenosis, and spinal nerve impingement, all of which may cause back pain. Additionally, injury to adjacent bony, ligamentous, or muscular structure may generate pain.
The primary goal of MRI imaging of the spine is to identify either a surgically correctable lesion or a process that could be treated by an epidural steroid injection.
Disc bulges and herniations are commonly seen in asymptomatic people. The high prevalence of these “abnormalities” complicates correlation of clinical and imaging findings.
Despite efforts to standardize the nomenclature with an estrablished lexicon, there is a fair amount of inter-observer variability in the reporting of degenerative spinal lesions.
Disc bulges and herniations
The normal intervertebral disc is composed of a central gelatinous nucleus pulposus and a fibrous peripheral annulus fibrosus. A disc bulge, or heniation, occurs when the substance of the disc (nucleus pulposus, annulus fibrosus, or both) extends beyond the disc’s normal margins.
A broad-based disc bulge disc bulge is present when >180º of the disc circumference extends beyond the expected margins of the disc. Technically, the bulge must measure greater than 2 mm over greater than 180º of its circumference. In practice, the radiologist makes this diagnosis subjectively and exact measurements are typically unnecessary.
A herniation is a focal disc bulge. Technically, a herniation must involve less than 90º of the disc circumference. In practice, measurements are rarely performed.
The term “herniation” is a nonspecific, medicolegally charged umbressla term taht is generally avoided in dictations. The preferred description for herniation is either protrusion or extrusion, depending on the morphology. In this text “herniation” refers to both protrusions and extrusions.
A protrusion is a focal herniation where the diameter of the neck is greater than the diameter of the dome.
An extrusion is a focal herniation where the diameter of the neck is less than the diameter of the dome. This is analogous to a saccular aneurysm.
Disc herniation may contribute to nerve root impingement and spinal stenosis.
Both the medial-lateral position and the spinal level of herniation dictate which nerve root is impinged upon.
In the thoracic and lumbar spine, each nerve root exits below its corresponding vertebral body. For instance, the L4 nerve root exits below the L4 vertebral body at L4-L5.
In contrast, in the cervical spine, each root exits above its corresponding vertebral body. For instance, the C7 nerve root exits above the C7 vertebral body at C6-C7. At the cervicothoracic transition, the C8 nerve root exits below the C7 vertebral body and the T1 nerve root exits below the T1 vertebral body.
Four terms describe the medial-lateral position of herniation: Central, paracentral (adjacent to the subarticular facet join), foraminal, and far-lateral (extra-foraminal). A medial (central or paracentral) herniation will affect the descending nerve root corresponding to the level below the disc. For instance, central, or paracentral herniation of the L4-L5 disc will impinge the descending L5 root. In contrast, a lateral (foraminal or far-lateral) herniation will affect the exiting nerve root, which would be L4 at the L4-5 level.
Once protruded, disc material may separate from its parent disc to become a sequestered fragment, which may subsequently migrate inferioly or superiorly. The surgeon needs to know about a sequestered fragment because it may preclude a minimally invasive procedure.

General degenerative changes
With aging, discs tend to become desiccated, manifested as T2 shortening (hypointensity on T2-weighted images).
Loss of disc space height may affect multiple levels.
Osteophytes can project anteriorly or posterioly. In the cervical spine, anterior osteophytes may cause dysphagia. Anywhere in the spine, posterioly projecting osteophytes may contribute to signal canal or neural foraminal stenosis.
A Schmorl’s node represents inferior or suprerior herniation of an intervertebral disc into the adjacent vertebral bony endplate, which creates a rounded bony defect at the endplate. An acute Schmorl’s node may be hyperintense on T2-weighted images and may contribute to back pain.
Secondary vertebral body changes (Modic changes)
Degenerative changes in the spine are associated with vertebral body endplate and subchondral marrow signal changes that were described and classified by Modic.
Modic type 1 changes are hyperintense on T2-weighted images and hypointense on T1-weighted images, reflecting bone marrow edema and inflammation. These changes may be associated with active back pain and their presence predicts a better outcome following lumbar spine surgery.
Modic type 2 changes are hyperintense on both T2- and T1-weighted images and reflect fatty proliferation within the affected marrow, thought to be secondary to chronic marrow ischemia. Modic 2 changes are less likely to be associated with active symptoms.
Modic type 3 changes are hypointense on both T2- and T1- weighted images and are thought to represent sclerosis. Their clinical significance is unclear.
Ligamentum flavum infolding/hypertrophy
The ligamentum flavum lines the lamina at the posterior aspect of the spinal canal. Thickening of the ligamentum flavum can contribute to spinal canal stenosis.
The terms “infolding”, “inward buckling”, and “hypertrophy” have been used to describe apparent thickening of the ligamentum flavum. The terms “infolding” or “inward buckling” are preferred by some authors as being more indicative of the actual process.
Facet arthropathy
The intervertebral facet joints are synovial joints that can undergo the same degenerative processes as larger synovial joints, including cartilage loss, osteophytosis, sclerosis, and subchondral cystic change.
The facet joints are located medial to the neural foramina. Degenerative changes of the facet joints (facet arthropathy) may contribute to neural foraminal narrowing.
A synovial cyst is an epidural cyst that is associated with facet arthropathy. On MRI, a synovial cyst is hyperintense on T2-weighted images and variable signal intensity on T1-weighted images depding on the presence of internal hemorrhage. Continuity of the synovial cyst with the facet joint may not be demonstrated on MRI, but epidural injection of the facet joint will show opacification of the cyst.
In contrast to a synovial cyst, a Tarlov cyst is not associated with facet arthropathy. A Tarlov cyst is a perineural cyst of the sacrum, formed within the nerve root sheath, and usually asymptomatic.
High intensity zone (annular fissure)
High intensity zone is the imaging term referring to an area of hyperintense signal on T2-weighted images in the annulus fibrosus. This imaging finding is thought to correlate with fissure or tear of the annulus fibrosus and may be a cause of pain.
The terms “annular fissure” and “annulus tear” are synonymous. Annular fissure is the preferred term as tear implies a traumatic etiology, while this process is typically due to disc degeneration.
Diffuse idiopathic skeletal hyperostosis (DISH)
Diffuse idiopathic skeletal hyperostosis (DISH) is flowing anterior osteophytes extending at least four vertebral levels. DISH and degenerative disc disease are separate processes, though they are often seen concurrently.
In contrast to discogenic degenerative disease, there is preservation of the disc spaces.
Similar to ankylosing spondylitis, DISH may predispose to spinal fracture after even minor trauma due to reduced mobility of the spinal column.
DISH is associated with ossification of the posterior longitudinal ligament.
Ossification of the posterior longitudinal ligament (OPLL)
Ossification of the posterior longitudinal ligament (OPLL) is calcification and ossification of the posterior longitudinal ligament, which may lead to spinal canal stenosis and compression of the anterior aspect of the cord.
OPLL is best appreciated on CT, where the abnormal ossification will be apparent in the anterior aspect of the spinal canal. MRI shows low signal in the anterior spinal canal.
OPLL begins in the cervical spine but may extend inferiorly to the thoracic spine.
Postoperative spine
Contrast is typically administered for postoperative MRI imaging of the spine to distinguish between recurrent disc disease and scar tissue.
Both disc and scar appear hypointense on T2-weighted MRI. In theory, scar tissue should enhance throughout, while recurrent disc diemonstrates only peripheral enhancement.
If there is recurrent disc herniation, further surgical intervention may be indicated. In general, however, the presence of scar tissue would decrease teh chance of successful relief of symptoms.
Pyogenic discitis/osteomyelitis
Pyogenic discitis/osteomyelitis is caused by bacterial infection of the intervertebral disc and adjacent vertebrae, typically from a hematogenous source. Staphylococcus aureus is the most common agent.
In adults, the vascularized subchondral bone is the initial site of infection, which spreads to the disc. In children, however, the intervertebral disc is initially infected, with subsequent spread to the vertebral endplates.
The key imaging appearance of discitis/osteomyelitis is marrow hypointensity on T1-weighted images centered on both sides of an abnormal intervertebral disc that is hyperintense on T2-weighted images. Loss of adjacent endplate definition is usually present. On radiography, the only initial clue may be loss of disc space height. Later in the course of infection, there may be vertebral collapse.
Adjacent soft tissue infection (paraspinal or epidural) is often present.
Tuberculous osteomyelitis
Also called Pott disease, tuberculous osteomyelitis represents infection of the vertebral body with Mycobacterium tuberculosis. Unlike pyogenic discitis/osteomyelitis, the discs are usually spared as M. tuberculosis lacks the proteolytic enzymes necessary to break down the disc substance.
Pott disease classically causes wedge-shaped compression of the anterior aspect of the vertebral body, often leading to a gibbus deformity centered at the infected vertebra. Gibbus deformity is an acutley angled kyphosis. Gibbus deformity may result from a vertebral compression fracture or can be seen in congenital syndromes including achondroplasia and the muccopolysaccharidoses (Hunter and Hurler syndromes.)
Approximately 10% of patients with Pott disease have active pulmonary tuberculosis.
Dural arteriovenous fistula (dAVF) spine
Dural arteriovenous fistula (dAVF) is the most common spinal vascular malformation. It typically affects older males with back pain and progressive myelopathy. Spinal dAVF is a Cognard type V dural AV fistula, as discussed in the section on CNS dAVFs.
MRI of dAVF shows flow voids surrouding the cord. The cord is often swollen, with abnormal intramedullary T2 prolongation. CT myelography shows serpiginous filling defects in the subarachnoid space.
Infarction (spine)
Spinal cord infarction is most common in the upper thoracic or thoracolumbar spine due to more precarious blood supply. The predominant blood supply to the distal cord is the artery of Adamkiewicz.
Spinal cord infarction clinically presents with loss of bowel and bladder control, loss of perineal sensation, and impairment in both motor and sensory function of the legs.
Risk factors for spinal cord infarction include aortic surgery (as in the case above), aortic aneurysm, arteritis, sickle cell anemia, vascular malformation, and disc herniation.
Imaging of spinal cord infarction shows hyperintensity of the affected cord regions on T2-weighted images. The cord may be enlarged. Diffusin images typically show restricted diffusion. Concomitant vertebral body infarction may be present, which is more common in sickle cell disease and chronic steroid use.
Tethered cord syndrome
Tethered cord syndrome is a clinical syndrome of back and leg pain, gait spasticity, and decreased lower extremity sensation.
Normally the conus should terminate superior to the inferior endplate of L2. If the conus terminates below this level, the cord may be tethered.
The cord may be tethered by a thickened filum or lipoma.
Diastematomyelia
Diastematomyelia is a congenitally split spinal cord, which may be a cause of scoliosis.
Fatty filum
Fat within the filum terminale may be associated with diastematomyelia, tethered cord, or may be clinically insignificant.


