NeuroBiology 31-65 Flashcards
- Which of the following statements about olfactory recep-tors is correct?
A. An olfactory receptor displays rapid adaptation initially
B. The life span of olfactory receptor cells is approxi-
mately 9 months
C. A single olfactory receptor cell typically responds to
only a single odorant
D. The receptor potential occurs when Na* channels are
closed in a manner similar to phototransduction
E. They are cGMP-regulated
A. An olfactory receptor displays rapid adaptation initially
Olfactory receptors (ORs) display rapid adaptation initially and little afterwards. Within the olfactory system, an olfactory stimulus results in the opening of sodium channels, which leads to depolarization and action potentials. These action potentials can increase in frequency to about 20/s. Adenylate cyclase activity catalyzes the formation of cAMP, resulting in opening of many additional channels, which can also increase the rate of discharge in olfactory neurons. Each olfactory neuron is capable of responding to many different odorants, as determined by electrophysiologic studies. The life span of ORs varies from 30 to 120 days in mammalian species. Replacement cells are delivered by mitosis of basal cells. The relatively rapid turnover of ORs makes them par-tially susceptible to damage after radiation therapy and/or chemotherapeutic agents, which target rapidly dividing cells (Kandel, pp. 626-636; Pritchard, pp. 266-267).
- Which of the following sensory systems sends signals directly to both the thalamus and cerebral cortex?
A. Two-point discrimination
B. Taste
C. Olfaction
D. Pain
E. Balance
C. Olfaction
Taste and sensation from the head are carried to
the ventroposterior medial (VPM) nucleus of the thalamus. Sensation and proprioception from the body reach the ventroposterior lateral (VPL) nucleus of the thalamus. The visual system utilizes the lateral geniculate nucleus (LGN) and the auditory system the medial geniculate nucleus (MGN) prior to being relayed to the cortex. Some olfactory information bypasses the thalamus to reach the orbitofrontal cortex, but it should be noted that some projections subserving smell can reach the orbitofrontal cortex via the mediodorsal (MD) thalamic nucleus. The olfactory system, therefore, relies on parallel processing to transmit olfactory inputs to the cortex (Kandel, p. 633).
- Cells most sensitive to radiation therapy
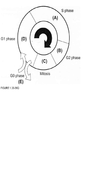
Directions: For each question select one or more than one lettered heading (in parentheses) from Figure 1.33-1.39Q with which it is most closely associated. Each lettered head-ing may be used once, more than once, or not at all.
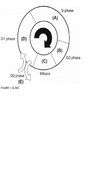
B,C
Cells are most sen-sitive to radiation during the G2 and M phases of the cell cycle and most resistant in the late S phase. Gl cells have intermediate sensitivity. The precise mechanism(s) accounting for these variations remains unclear, but studies have shown that differences in a cell’s ability to repair DNA damage in different phases after radiation may play an important part. In the Gl phase of the cycle, the nucleus has a diploid amount of DNA (2G), which increases to 4C by the end of the S phase. Only cells in the S phase (DNA synthetic phase) are able to incorporate thymidine analogues (bro-modeoxyuridine) into their nuclear DNA. Nutrient depletion and crowding can result in the movement of cells into the quiescent or nonproliferating phase (GO) of the cell cycle; such cells can eventually re-enter the cell cycle at a later point in time. Mitosis is the most easily identifiable stage of the cell cycle by light microscopy. The genes encoding pl6 (CDKN2A) and pl5 (CDKN2B) map onto chromosome 9p21, a site that is associated with homozygous deletions in high-grade astrocytomas in about two-thirds of gliomas. These proteins act as inhibitors of cyclin-dependent kinases and other pathways during the Gl phase of the cell cycle and help control proliferation at the Gl/S phase of the cell cycle. The TP53 protein assists in several cellular processes, including cell cycle regulation, response of cells to DNA damage (Psr dependent growth arrest following DNA damage occurs in Gl phase of the cell cycle), cell death, cell differentiation, and neovascularization (WHO, pp. 11-14 ; Berger, pp. 204-209) .
- Nutrient depletion or physical crowding are conditions that encourage cells to move into this phase of the cell cycle
For each question select one or more than one lettered heading (in parentheses) from Figure 1.33-1.39Q with which it is most closely associated. Each lettered head-ing may be used once, more than once, or not at all.
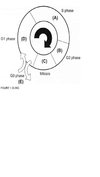
E
Cells are most sen-sitive to radiation during the G2 and M phases of the cell cycle and most resistant in the late S phase. Gl cells have intermediate sensitivity. The precise mechanism(s) accounting for these variations remains unclear, but studies have shown that differences in a cell’s ability to repair DNA damage in different phases after radiation may play an important part. In the Gl phase of the cycle, the nucleus has a diploid amount of DNA (2G), which increases to 4C by the end of the S phase. Only cells in the S phase (DNA synthetic phase) are able to incorporate thymidine analogues (bro-modeoxyuridine) into their nuclear DNA. Nutrient depletion and crowding can result in the movement of cells into the quiescent or nonproliferating phase (GO) of the cell cycle; such cells can eventually re-enter the cell cycle at a later point in time. Mitosis is the most easily identifiable stage of the cell cycle by light microscopy. The genes encoding pl6 (CDKN2A) and pl5 (CDKN2B) map onto chromosome 9p21, a site that is associated with homozygous deletions in high-grade astrocytomas in about two-thirds of gliomas. These proteins act as inhibitors of cyclin-dependent kinases and other pathways during the Gl phase of the cell cycle and help control proliferation at the Gl/S phase of the cell cycle. The TP53 protein assists in several cellular processes, including cell cycle regulation, response of cells to DNA damage (Psr dependent growth arrest following DNA damage occurs in Gl phase of the cell cycle), cell death, cell differentiation, and neovascularization (WHO, pp. 11-14 ; Berger, pp. 204-209) .
- Cells can incorporate thymidine analogues into their
nuclear DNA
For each question select one or more than one lettered heading (in parentheses) from Figure 1.33-1.39Q with which it is most closely associated. Each lettered head-ing may be used once, more than once, or not at all.

A
Cells are most sen-sitive to radiation during the G2 and M phases of the cell cycle and most resistant in the late S phase. Gl cells have intermediate sensitivity. The precise mechanism(s) accounting for these variations remains unclear, but studies have shown that differences in a cell’s ability to repair DNA damage in different phases after radiation may play an important part. In the Gl phase of the cycle, the nucleus has a diploid amount of DNA (2G), which increases to 4C by the end of the S phase. Only cells in the S phase (DNA synthetic phase) are able to incorporate thymidine analogues (bro-modeoxyuridine) into their nuclear DNA. Nutrient depletion and crowding can result in the movement of cells into the quiescent or nonproliferating phase (GO) of the cell cycle; such cells can eventually re-enter the cell cycle at a later point in time. Mitosis is the most easily identifiable stage of the cell cycle by light microscopy. The genes encoding pl6 (CDKN2A) and pl5 (CDKN2B) map onto chromosome 9p21, a site that is associated with homozygous deletions in high-grade astrocytomas in about two-thirds of gliomas. These proteins act as inhibitors of cyclin-dependent kinases and other pathways during the Gl phase of the cell cycle and help control proliferation at the Gl/S phase of the cell cycle. The TP53 protein assists in several cellular processes, including cell cycle regulation, response of cells to DNA damage (Psr dependent growth arrest following DNA damage occurs in Gl phase of the cell cycle), cell death, cell differentiation, and neovascularization (WHO, pp. 11-14 ; Berger, pp. 204-209) .
- Cells most resistant to radiation therapy
For each question select one or more than one lettered heading (in parentheses) from Figure 1.33-1.39Qwith which it is most closely associated. Each lettered head-ing may be used once, more than once, or not at all.
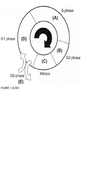
A
Cells are most sen-sitive to radiation during the G2 and M phases of the cell cycle and most resistant in the late S phase. Gl cells have intermediate sensitivity. The precise mechanism(s) accounting for these variations remains unclear, but studies have shown that differences in a cell’s ability to repair DNA damage in different phases after radiation may play an important part. In the Gl phase of the cycle, the nucleus has a diploid amount of DNA (2G), which increases to 4C by the end of the S phase. Only cells in the S phase (DNA synthetic phase) are able to incorporate thymidine analogues (bro-modeoxyuridine) into their nuclear DNA. Nutrient depletion and crowding can result in the movement of cells into the quiescent or nonproliferating phase (GO) of the cell cycle; such cells can eventually re-enter the cell cycle at a later point in time. Mitosis is the most easily identifiable stage of the cell cycle by light microscopy. The genes encoding pl6 (CDKN2A) and pl5 (CDKN2B) map onto chromosome 9p21, a site that is associated with homozygous deletions in high-grade astrocytomas in about two-thirds of gliomas. These proteins act as inhibitors of cyclin-dependent kinases and other pathways during the Gl phase of the cell cycle and help control proliferation at the Gl/S phase of the cell cycle. The TP53 protein assists in several cellular processes, including cell cycle regulation, response of cells to DNA damage (Psr dependent growth arrest following DNA damage occurs in Gl phase of the cell cycle), cell death, cell differentiation, and neovascularization (WHO, pp. 11-14 ; Berger, pp. 204-209) .
- P15 and pl6 cause growth arrest in this cell-cycle phase
For each question select one or more than one lettered heading (in parentheses) from Figure 1.33-1.39Q with which it is most closely associated. Each lettered head-ing may be used once, more than once, or not at all.
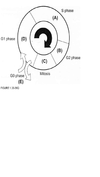
D
Cells are most sen-sitive to radiation during the G2 and M phases of the cell cycle and most resistant in the late S phase. Gl cells have intermediate sensitivity. The precise mechanism(s) accounting for these variations remains unclear, but studies have shown that differences in a cell’s ability to repair DNA damage in different phases after radiation may play an important part. In the Gl phase of the cycle, the nucleus has a diploid amount of DNA (2G), which increases to 4C by the end of the S phase. Only cells in the S phase (DNA synthetic phase) are able to incorporate thymidine analogues (bro-modeoxyuridine) into their nuclear DNA. Nutrient depletion and crowding can result in the movement of cells into the quiescent or nonproliferating phase (GO) of the cell cycle; such cells can eventually re-enter the cell cycle at a later point in time. Mitosis is the most easily identifiable stage of the cell cycle by light microscopy. The genes encoding pl6 (CDKN2A) and pl5 (CDKN2B) map onto chromosome 9p21, a site that is associated with homozygous deletions in high-grade astrocytomas in about two-thirds of gliomas. These proteins act as inhibitors of cyclin-dependent kinases and other pathways during the Gl phase of the cell cycle and help control proliferation at the Gl/S phase of the cell cycle. The TP53 protein assists in several cellular processes, including cell cycle regulation, response of cells to DNA damage (Psr dependent growth arrest following DNA damage occurs in Gl phase of the cell cycle), cell death, cell differentiation, and neovascularization (WHO, pp. 11-14 ; Berger, pp. 204-209) .
- TP53-dependent growth arrest following DNA damage occurs in this phase
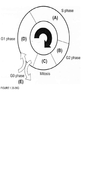
For each question select one or more than one lettered heading (in parentheses) from Figure 1.33-1.39Q with which it is most closely associated. Each lettered head-ing may be used once, more than once, or not at all
D
Cells are most sen-sitive to radiation during the G2 and M phases of the cell cycle and most resistant in the late S phase. Gl cells have intermediate sensitivity. The precise mechanism(s) accounting for these variations remains unclear, but studies have shown that differences in a cell’s ability to repair DNA damage in different phases after radiation may play an important part. In the Gl phase of the cycle, the nucleus has a diploid amount of DNA (2G), which increases to 4C by the end of the S phase. Only cells in the S phase (DNA synthetic phase) are able to incorporate thymidine analogues (bro-modeoxyuridine) into their nuclear DNA. Nutrient depletion and crowding can result in the movement of cells into the quiescent or nonproliferating phase (GO) of the cell cycle; such cells can eventually re-enter the cell cycle at a later point in time. Mitosis is the most easily identifiable stage of the cell cycle by light microscopy. The genes encoding pl6 (CDKN2A) and pl5 (CDKN2B) map onto chromosome 9p21, a site that is associated with homozygous deletions in high-grade astrocytomas in about two-thirds of gliomas. These proteins act as inhibitors of cyclin-dependent kinases and other pathways during the Gl phase of the cell cycle and help control proliferation at the Gl/S phase of the cell cycle. The TP53 protein assists in several cellular processes, including cell cycle regulation, response of cells to DNA damage (Psr dependent growth arrest following DNA damage occurs in Gl phase of the cell cycle), cell death, cell differentiation, and neovascularization (WHO, pp. 11-14 ; Berger, pp. 204-209) .
- Most variable phase of the cell cycle in terms of duration
For each question select one or more than one lettered heading (in parentheses) from Figure 1.33-1.39Q with which it is most closely associated. Each lettered head-ing may be used once, more than once, or not at all
D
Cells are most sen-sitive to radiation during the G2 and M phases of the cell cycle and most resistant in the late S phase. Gl cells have intermediate sensitivity. The precise mechanism(s) accounting for these variations remains unclear, but studies have shown that differences in a cell’s ability to repair DNA damage in different phases after radiation may play an important part. In the Gl phase of the cycle, the nucleus has a diploid amount of DNA (2G), which increases to 4C by the end of the S phase. Only cells in the S phase (DNA synthetic phase) are able to incorporate thymidine analogues (bro-modeoxyuridine) into their nuclear DNA. Nutrient depletion and crowding can result in the movement of cells into the quiescent or nonproliferating phase (GO) of the cell cycle; such cells can eventually re-enter the cell cycle at a later point in time. Mitosis is the most easily identifiable stage of the cell cycle by light microscopy. The genes encoding pl6 (CDKN2A) and pl5 (CDKN2B) map onto chromosome 9p21, a site that is associated with homozygous deletions in high-grade astrocytomas in about two-thirds of gliomas. These proteins act as inhibitors of cyclin-dependent kinases and other pathways during the Gl phase of the cell cycle and help control proliferation at the Gl/S phase of the cell cycle. The TP53 protein assists in several cellular processes, including cell cycle regulation, response of cells to DNA damage (Psr dependent growth arrest following DNA damage occurs in Gl phase of the cell cycle), cell death, cell differentiation, and neovascularization (WHO, pp. 11-14 ; Berger, pp. 204-209) .
- What is the resting membrane potential for nerve cells?
A. -100 mV
B. -90 mV
C. -80 mV
D. -65 mV
E. -40 mV
D. -65 mV
In resting nerve cells the resting membrane potential
is -65 mV. This negative polarity is largely the result of two factors: the selective permeability of the cell membrane to K+ through voltage-gated channels and the Na+, K+pump, which pumps three Na+ions out of the cell for every two K+ions that are pumped inside.
In terms of K permeability, as K+leaks out of the cell
down its concentration gradient, the cell membrane begins to develop a potential difference due to the accumulation of negative charges inside the cell. This eventually slows the continued efflux of K+ions out of the cell as a result of the electrostatic attraction between the inside of the cell and posi-tively charged K+ions outside the cell. Eventually the rate of
K+flow inside and outside the cell reaches a state of equilib-rium (equilibrium potential for K+) due to the balancing of the electrical and chemical forces. This produces a net flow of K+ions that is zero and a net negative potential difference across the cell membrane. This is called the equilibrium
potential for K+ and can be calculated by the Nernst equation.
E = RT/F log(ion)out/(ion)i n= 61 log (150/5.5) = -86 mV
Using standard values of concentration gradients (see dis-cussion question 41, RT/F = 61), the equilibrium potential forK+ is -86 mV, which would also be the resting membrane potential across the cell membrane if K+ were the only ion contributing to the membrane potential. However, rarely
does one ion contribute solely to the membrane potential,which is often a combination of multiple ions diffusing through the membrane. For this reason, the Goldman equa-tion was developed to account for the relationship between membrane potential (V) and relative permeability (P) of each population of ion channels. Given this, the resting membrane potential in neurons (-65 mV) is not identical to E K+ (-86 mV), since the membrane is slightly permeable to other ions as well.
v = 61j PK(K+)„u,+ PNa+(Na+)ol„+Pa(Gl-)in
<sup>8</sup>P<sub>K<sup>+</sup></sub>(K<sup>+</sup>)<sub>jn</sub> + P<sub>X;l<sup>+</sup></sub>(Na<sup>+</sup>)<sub>jn </sub>+ P<sub>a</sub>(Cn<sub>out</sub>
The inequality of charge on either side of the cell mem-brane is also the result of the Na+, K+ pump, which is a large membrane-spanning protein with Na+, K+, and ATP binding sites. If this pump were not present, the gradient across the cell membrane would eventually dissipate. This pump utilizes one ATP molecule to pump 3 Na+ ions out of and 2 K+ ions into the cell. An increase in permeability of Gl” channels usually has little effect on membrane potential, since the resting potential of a typical neuron (-65 mV) and equilib-rium potential for Gl” (-66 mV) are very similar (Kandel,pp. 125-139).
- What is the extracellular concentration of Ca2+
ions in the brain?
A. 0.7 mM/L
B. 2 mM/L,
C. 125 mM/L
D. 150 mM/L
E. None of the above
B. 2 mM/L,

Refer to Table 1.41A. Neurons maintain a high con-centration of K+ ions and organic anions inside the cell, and ions such as Na+ , Gl”, and Ga2+ are more highly concentrated outside of the cell (Kandel, pp. 125-139).
- Columns of neurons in area 3a of the somatic sensory cortex receive input primarily from what type of receptor(s)?
- Rapidly adapting skin receptors
- Slowly and rapidly adapting skin receptors
- Pressure and joint position receptors
- Muscle stretch receptors
A. 1,2, and 3 are correct
B. 1 and 3 are correct
C. 2 and 4 are correct
D. Only 4 is correct
E. All of the above
D

- Which of the following is true of action potentials?
- Action potentials are mediated entirely by changes in K+ voltage-gated channels
- The rate of Na+ influx begins to slow as the membrane potential approaches EK+
- The threshold for initiating action potentials is usually around +15 mV
- The falling phase of the action potential is mediated by delayed activation of K+ conductance
A. 1, 2, and 3 are correct
B. 1 and 3 are correct
C. 2 and 4 are correct
D. Only 4 is correct
E. All of the above
D. Only 4 is correct
The rising phase of an action potential is due to a
stimulus that results in the activation of voltage-gated Na+ channels. The rate of Na+ influx begins to slow as the mem-brane reaches the membrane potential for Na+ (not K+),resulting in a peak amplitude when the Na+ channels become inactivated. The decline in the action potential is then medi-ated by the delayed activation of voltage-gated K+ channels. The efflux of K+ ions is greatest at the peak of the action potential and begins to decline as the membrane potential approaches the equilibrium potential for K+ . The membrane is, however, briefly hyperpolarized, as K+ conductance does not return to resting levels until after the membrane voltage has declined below the normal resting potential. The thresh-old for initiating action potentials may vary but is usually around -50 mV for most mammalian neurons, not +15 mV (Kandel, pp. 150-170; Pritchard, pp. 23-25).
- Cells with concentric receptive fields along the visual
pathway are found in what location(s)?
A. Retina
B. Retina and optic nerve
C. Retina and lateral geniculate nucleus
D. Retina, lateral geniculate nucleus, layer 4 of the visual
cortex
E. Cells in the premotor cortex only
C. Retina and lateral geniculate nucleus
Both ganglion cells in the retina and the lateral
geniculate nucleus are known to have both “on-center” and “off-surround,” or concentric, receptive fields. Cells in the optic nerve and premotor cortex are not known to possess such characteristics. Simple cells in layer IV of the visual cortex do not have circular receptive fields but instead respond to stimuli as lines and bars (rectangles) (Kandel,pp. 517-522, 528-529).
- What is the primary neurotransmitter of the Renshaw cell?
A. Glycine
B. Acetylcholine
C. GABA
D. Serotonin
E. Glutamate
A. Glycine
A special class of inhibitory interneurons called Renshaw cells are found in laminae MI and VIII of the spinal cord. These cells have muscarinic cholinergic receptors that receive oc-motor-neuron cholinergic collateral projections. The Renshaw cell then exerts a negative feedback on the a motor neuron and other homonymous a motor neurons, called recurrent inhibition. The neurotransmitter released by Renshaw cells is glycine. Renshaw cells also make inhibi-tory synaptic connections with la inhibitory interneurons; this arrangement regulates reciprocal inhibition of antago-nistic motor neurons. Renshaw cells receive input from several descending pathways in the spinal cord (Carpenter,pp. 57-79; Kandel, pp. 720-721).
- A patient with homonymous hemianopsia due to a
parietal lesion will have deficient pursuit eye movements_________of the lesion, resulting in opticokinetic nystagmus. The opticokinetic nystagmus will be decreased when the drum is rotated________the side of the lesion.
A. Opposite the side, toward
B. Toward the side, away from
C. Opposite the side, away from
D. Toward the side, toward
E. None of the above
D. Toward the side, toward
The precise pathways of the opticokinetic system
remain unclear but are believed to be similar to smooth pursuits. The pathway is believed to extend from the visual association areas (18 and 19) to the horizontal gaze center of the abducens nucleus in the pons. The pathway from the left visual association area is believed to terminate in the left pontine gaze center, resulting in pursuit movement of the eyes to the left. Similarly, the right visual association region produces movements to the right. A patient with a pure occipital lobe lesion theoretically should have no difficulty with pursuits, since the pathways originate in more anterior regions. The opticokinetic response should, therefore, be symmetric. A patient with homonymous hemianopsia and a parietal lesion will have deficient pursuit movements to the same side of the lesion, resulting in an asymmetric opti-cokinetic response (OKN). The opticokinetic response will be decreased when the drum is rotated toward the side of the lesion. Patients with homonymous hemianopsia due to either an optic tract, temporal lobe, or purely occipital lobe lesions should have symmetric opticokinetic responses to both sides. Cogum’s dictum can be used to summarize these findings. Homonymous hemianopsia + asymmetric OKN is most likely related to a parietal mass lesion. Homonymous hemianopia + symmetric OKN is most likely a result of an occipital lesion such as stroke (Kline, pp. 16-17).
- All of the following biochemical features regarding re-ceptors for chemical neurotransmitters are correct EXCEPT?
A. They may be membrane-spanning proteins
B. They can work in a direct or indirect fashion to
influence synaptic response
C. They can influence cells by activating second messen-gers, such as cAMP or diacylglycerol
D. They can help reinforce the pathways involved with
learning
E. The binding site on the nicotinic acetylcholine recep-tor usually includes both the a and P subunits
E. The binding site on the nicotinic acetylcholine recep-tor usually includes both the a and P subunits
Direct receptors like nicotinic ACh receptors are also
referred to as ionotropic receptors, which gate ionic current rapidly over only a few milliseconds. The ACh receptor itself is a transmembrane protein composed of five subunits (a2(3y5) with the a subunits representing the binding site for ACh. Receptors that gate ion channels indirectly are called metabotropic receptors and typically produce slower synap-tic responses lasting seconds to minutes. Activation of these receptors often requires the production of second messen-gers such as cAMP and diacylglycerol, ultimately resulting in the modulation of ion channels distinct from the receptor itself. Noradrenergic and serotonergic receptors are exam-ples of indirect receptors. The metabotropic receptors have been shown to influence learning and modulate behavior (Kandel, p. 185).
- All of the following statements about the semicircular canals are correct EXCEPT?
A. The movement of endolymph within each canal is
opposite to the direction of head rotation
B. Primary afferent fibers do not discharge after head
rotation ceases
C. Linear acceleration of the head is sufficient to activate the posterior semicircular canal
D. The floor of the ampulla contains a ridge of specialized hair cells that is covered by a layer of gelatin called the cupula
E. Hair cells in the horizontal canal are polarized toward the utricle, and those in the anterior and posterior semicircular canals are polarized away from the utricle
C. Linear acceleration of the head is sufficient to activate the posterior semicircular canal

Refer to Figure 1.48A. One end of each semicircular
canal contains an enlarged region known as the ampulla, where the flow of endolymph serves as a mechanical stimu-lus for sensory transduction. The floor of the ampulla con-tains specialized hair cells, the crista ampullaris, and is covered by a gelatinous layer known as the cupula. The ster-eocilia of the hair cells insert into the cupula. These hair cells are stimulated by changes in endolymph circulation induced by head rotation. The movement of endolymph within each canal is opposite to the direction of head rotation. The response in each pair of semicircular canals (one on each side of the head) is opposite as well. Rotation of the head or angular acceleration is sufficient to stimulate a response in the semicircular canals but insufficient to stimulate the macula of the utricle, which requires linear acceleration. Firing typically ceases once head movement stops. Hair cells in the horizontal canal are polarized toward the utricle, and those in the anterior and posterior semicircular canals are polarized away from the utricle (Kandel, pp. 802-806;Pritchard, pp. 250-253).
- Slow synaptic transmission between nociceptors and
dorsal horn neurons is mediated primarily by what neuro-transmitter?
A. Substance P
B. Glutamate
C. Acetylcholine
D. ATP
E. Serotonin
A. Substance P
A. Slow-excitatory synaptic transmission between no-ciceptors and dorsal horn neurons in the marginal layer of lamina I and substantia gelatinosa of lamina II is mediated primarily by substance P, released by A5 and G fibers (Kandel, pp. 477-479).
- A motor unit is composed of
A. A group of a motor neurons to a given muscle
B. A group of a and y motor neurons to a given muscle
C. A group of a motor neurons to a given muscle and all of the muscle fibers they innervate
D. A group of muscle fibers innervated by a single motor
neuron
E. All muscle groups innervated by the ventral root
D. A group of muscle fibers innervated by a single motor
neuron
The motor unit is the functional unit of muscle
contraction; it includes a single motor neuron and all of the muscle fibers it innervates (Kandel, p. 81)
- Group lb sensory fibers from muscle are most sensitive to what sensory modality?
- Muscle length
- Deep pressure
- Rate of change in length
- Muscle tension
A. 1, 2, and 3 are correct
B. 1 and 3 are correct
C. 2 and 4 are correct
D. Only 4 is correct
E. All of the above are correct
D. Only 4 is correct
Refer to Table 1.51A. Sensory fibers from muscle are
typically classified according to their diameter. Group la sen-sory fibers (annulospiral endings and flower-spray endings) are between 12 to 20 um in diameter, myelinated, sensitive to muscle length and rate of change in length, and receive their input from muscle spindles. Group lb fibers are similar in diameter to group la, are also myelinated, and are most sensitive to muscle tension from Golgi tendon organs. Group II sensory fibers receive their input from secondary spindle endings and nonspindle endings and are between 6 to 12 um in diameter. Secondary spindle endings are sensitive to muscle length and nonspindle endings are sensitive to deep pressure. Group III sensory fibers receive input from free nerve endings, are between 2 to 6 um in diameter, and are responsive to pain as well as chemical and temperature stimuli. Type IV sensory endings are similar to type III with the exception of being smaller in diameter (0.5 to 2 um). Intrafusal fibers of muscles spindles are in parallel with extrafusal muscle fibers, whereas Golgi tendon organs (GTOs) are connected in series to skeletal muscle fibers, innervated by lb sensory afferents, and sensitive to muscle tension, as described above (Kandel, pp. 720-723)
- Which of the following is a component of the muscle
spindle? - Intrafusal muscle fibers
- Annulospiral endings
- Flower-spray endings
- y motor fibers
A. 1, 2, and 3 are correct
B. 1 and 3 are correct
C. 2 and 4 are correct
D. Only 4 is correct
E. All of the above
E. All of the above
Refer to Figure 1.52A. Muscle spindles are the sen-sory receptors of skeletal muscle that signal changes in
muscle length. Changes in muscle length are closely associ-ated with changes in the angles of the joints that the muscles cross; thus muscle spindles are capable of sensing relative positions of various body segments. The main components of the muscle spindle include intrafusal muscle fibers with noncontractile central regions, afferent sensory endings originating from the center of the intrafusal fibers (flower-spray and annulospiral nerve endings), and efferent motor fibers (static and dynamic y motor neurons) (Kandel, pp. 718 -719).
- Striking the ligamentum patellae with a reflex hammer results in the activation of which of the following struc-ture^)?
- Annulospiral endings
- Flower spray endings
- a motor neurons
- Quadriceps muscle
A. 1, 2, and 3 are correct
B. 1 and 3 are correct
C. 2 and 4 are correct
D. Only 4 is correct
E. All of the above are correct
E. All of the above are correct

Refer to Figure 1.52A. Striking the ligamentum patel-lae results in stretching of the intrafusal muscle spindles of the quadriceps muscle. In turn, this causes activation of both annulospiral and flower-spray sensory endings (responsive to stretching around the central region of intrafusal muscle fibers), which are carried to the dorsal horn of the spinal cord within the femoral nerve (L 2, 3, 4). These afferent fibers synapse with large a motor neurons in the anterior gray horns of the spinal cord. Nerve impulses then travel via efferent a motor neurons of the femoral nerve and stimulate the extrafusal fibers of the quadriceps muscle, which con-tracts. The motor neurons of the antagonist muscles are inhibited. After the muscle contracts, there comes a point at which the intrafusal muscle fibers slacken and are unable to signal any further changes in muscle length, which results in a decreased amount of firing of the afferent sensory fibers (annulospiral and flower spray). At this point, one role of y motor fibers is to maintain tension on muscle spindle poles during muscle contraction to ensure their firing during movement. The y motor neurons accomplish this task by terminating as small branches on motor endplates located on both ends of the intrafusal muscle fibers. Stimulation of these motor nerves causes the ends of the intrafusal fibers to contract, which in turn activates sensory endings. Thus, the y motor neurons provide a mechanism for adjusting the sen-sitivity of the muscle spindles to keep them under constant tension during muscle movement. In many voluntary move-ments, the y motor neurons are activated at the same time as a motor neurons to automatically maintain a level of spindle loading. This is called alpha-gamma coactivation. Under rest-ing conditions, the muscle spindles give rise to afferent nerve impulses at a constant rate, which is not consciously per-ceived. Although the details remain unclear, it is believed that this constant baseline firing of muscle spindles helps maintain tone (Kandel, pp. 713-736)
- Which of the following statements about neurons is
correct?
A. Golgi type I neurons form the short fiber tracts of the
brain and spinal cord
B. Golgi type II neurons have long axons that terminate in
the neighborhood of the cell body
C. Golgi type I neurons are inhibitory
D. The volume of cytoplasm within the cell body always
exceeds that found in the neurites
E. Golgi type II neurons greatly outnumber type I neurons
E. Golgi type II neurons greatly outnumber type I neurons
Golgi type I axons are typically long and include the
pyramidal cells of the cerebral cortex, the Purkinje cells
of the cerebellar cortex, and the motor cells of the spinal
cord. Golgi type II neurons have shorter axons, greatly out-number type I neurons, and are usually inhibitory. They have short dendrites, which gives them a star-shaped appear-ance. The volume of cytoplasm in the axons and dendrites usually exceeds the volume in the cell body (Bear, p. 41;Carpenter, pp. 65,126, 214, 229, 233, 330, 390, 395)
- Retrograde transport
A. Kinesin
B. Dynein
C. Dynamin
D. None of the above
E. All of the above
B. Dynein
Membranous organelles and secretory vesicles are transported to the axon terminal via fast anterograde axonal transport. This mode of transport is dependent on the protein kinesin and ATP and occurs at a rate of > 400 mm/day. Kinesin binds the organelle or vesicle and then forms intermittent cross bridges with tracks of microtubules, resulting in stepwise transport down the axon. The pharmacologic agents vinblastine and colchicine bind to and interfere with microtubule structure (not kinesin), thereby disrupting fast anterograde transport. There are several types of slow anterograde axonal trans-port. Component A utilizes a protein called dynamin, is GTP-dependent, and facilitates transport of cytosolic proteins and cytoskeletal elements. It is much slower than fast antero-grade transport, occurring at a rate of 0.2 to 2.5 mm/day. Component B is slightly faster, at 2 to 4 mm/day, and utilizes an actin/myosin motor complex in the transport of cytosolic proteins, actin, and spectrin. Fast retrograde axonal transport occurs at a rate of > 400 mm/day and is dependent on the protein dynein and hydrolysis of ATP. Retrograde transport facilitates the passage of endosomes from the axon terminal to the neuron soma. Endosomes contain various proteins (such as nerve growth factor) and even pathogens (such as rabies virus or tetanus toxin) that are taken up by the axon terminal via endocytosis (Kandel, pp. 99-103) .
- Fast anterograde transport
A. Kinesin
B. Dynein
C. Dynamin
D. None of the above
E. All of the above
A. Kinesin
Membranous organelles and secretory vesicles are transported to the axon terminal via fast anterograde axonal transport. This mode of transport is dependent on the protein kinesin and ATP and occurs at a rate of > 400 mm/day. Kinesin binds the organelle or vesicle and then forms intermittent cross bridges with tracks of microtubules, resulting in stepwise transport down the axon. The pharmacologic agents vinblastine and colchicine bind to and interfere with microtubule structure (not kinesin), thereby disrupting fast anterograde transport. There are several types of slow anterograde axonal trans-port. Component A utilizes a protein called dynamin, is GTP-dependent, and facilitates transport of cytosolic proteins and cytoskeletal elements. It is much slower than fast antero-grade transport, occurring at a rate of 0.2 to 2.5 mm/day. Component B is slightly faster, at 2 to 4 mm/day, and utilizes an actin/myosin motor complex in the transport of cytosolic proteins, actin, and spectrin. Fast retrograde axonal transport occurs at a rate of > 400 mm/day and is dependent on the protein dynein and hydrolysis of ATP. Retrograde transport facilitates the passage of endosomes from the axon terminal to the neuron soma. Endosomes contain various proteins (such as nerve growth factor) and even pathogens (such as rabies virus or tetanus toxin) that are taken up by the axon terminal via endocytosis (Kandel, pp. 99-103) .
- Slow anterograde transport
A. Kinesin
B. Dynein
C. Dynamin
D. None of the above
E. All of the above
C. Dynamin
Membranous organelles and secretory vesicles are transported to the axon terminal via fast anterograde axonal transport. This mode of transport is dependent on the protein kinesin and ATP and occurs at a rate of > 400 mm/day. Kinesin binds the organelle or vesicle and then forms intermittent cross bridges with tracks of microtubules, resulting in stepwise transport down the axon. The pharmacologic agents vinblastine and colchicine bind to and interfere with microtubule structure (not kinesin), thereby disrupting fast anterograde transport. There are several types of slow anterograde axonal trans-port. Component A utilizes a protein called dynamin, is GTP-dependent, and facilitates transport of cytosolic proteins and cytoskeletal elements. It is much slower than fast antero-grade transport, occurring at a rate of 0.2 to 2.5 mm/day. Component B is slightly faster, at 2 to 4 mm/day, and utilizes an actin/myosin motor complex in the transport of cytosolic proteins, actin, and spectrin. Fast retrograde axonal transport occurs at a rate of > 400 mm/day and is dependent on the protein dynein and hydrolysis of ATP. Retrograde transport facilitates the passage of endosomes from the axon terminal to the neuron soma. Endosomes contain various proteins (such as nerve growth factor) and even pathogens (such as rabies virus or tetanus toxin) that are taken up by the axon terminal via endocytosis (Kandel, pp. 99-103) .
- GTP-dependent
A. Kinesin
B. Dynein
C. Dynamin
D. None of the above
E. All of the above
C. Dynamin
Membranous organelles and secretory vesicles are transported to the axon terminal via fast anterograde axonal transport. This mode of transport is dependent on the protein kinesin and ATP and occurs at a rate of > 400 mm/day. Kinesin binds the organelle or vesicle and then forms intermittent cross bridges with tracks of microtubules, resulting in stepwise transport down the axon. The pharmacologic agents vinblastine and colchicine bind to and interfere with microtubule structure (not kinesin), thereby disrupting fast anterograde transport. There are several types of slow anterograde axonal trans-port. Component A utilizes a protein called dynamin, is GTP-dependent, and facilitates transport of cytosolic proteins and cytoskeletal elements. It is much slower than fast antero-grade transport, occurring at a rate of 0.2 to 2.5 mm/day. Component B is slightly faster, at 2 to 4 mm/day, and utilizes an actin/myosin motor complex in the transport of cytosolic proteins, actin, and spectrin. Fast retrograde axonal transport occurs at a rate of > 400 mm/day and is dependent on the protein dynein and hydrolysis of ATP. Retrograde transport facilitates the passage of endosomes from the axon terminal to the neuron soma. Endosomes contain various proteins (such as nerve growth factor) and even pathogens (such as rabies virus or tetanus toxin) that are taken up by the axon terminal via endocytosis (Kandel, pp. 99-103) .
- Binds vinblastine and colchicine to inhibit fast antero-grade transport
A. Kinesin
B. Dynein
C. Dynamin
D. None of the above
E. All of the above
D. None of the above
Membranous organelles and secretory vesicles are transported to the axon terminal via fast anterograde axonal transport. This mode of transport is dependent on the protein kinesin and ATP and occurs at a rate of > 400 mm/day. Kinesin binds the organelle or vesicle and then forms intermittent cross bridges with tracks of microtubules, resulting in stepwise transport down the axon. The pharmacologic agents vinblastine and colchicine bind to and interfere with microtubule structure (not kinesin), thereby disrupting fast anterograde transport. There are several types of slow anterograde axonal trans-port. Component A utilizes a protein called dynamin, is GTP-dependent, and facilitates transport of cytosolic proteins and cytoskeletal elements. It is much slower than fast antero-grade transport, occurring at a rate of 0.2 to 2.5 mm/day. Component B is slightly faster, at 2 to 4 mm/day, and utilizes an actin/myosin motor complex in the transport of cytosolic proteins, actin, and spectrin. Fast retrograde axonal transport occurs at a rate of > 400 mm/day and is dependent on the protein dynein and hydrolysis of ATP. Retrograde transport facilitates the passage of endosomes from the axon terminal to the neuron soma. Endosomes contain various proteins (such as nerve growth factor) and even pathogens (such as rabies virus or tetanus toxin) that are taken up by the axon terminal via endocytosis (Kandel, pp. 99-103) .
- All of the following are true about GABA-responsive channels EXCEPT?
A. The GABA, receptor consists of five subunits (0C2P2Y)
B. Picrotoxin inhibits the GABA ,receptor after binding
to the p subunit
C. The GABA,, receptor increases K+ conductance and generates an inhibitory postsynaptic potential (IPSP) after binding baclofen
D. The (3 subunit of the GABA receptor binds benzo-diazepines
E. The binding of alcohol, barbiturates, or benzodiazepines to the GABA receptor increases CP conductance
D. The (3 subunit of the GABA receptor binds benzo-diazepines
GABAA receptors consist of five subunits: two a, two
P, and one y subunit (a2P2y). All three subunits bind GABA, while the a and P subunits bind barbiturates and the y subunit binds benzodiazepines. After binding GABA, the channel opens and permits the entry of Gl” into the cell, generating
an inhibitory postsynaptic potential (IPSP). The binding of neuromodulators such as alcohol, barbiturates, and benzodi-azepines increases the GABA-induced Gl” current but does not open the channel directly. Picrotoxin inhibits the GABAA receptor after binding to the [3 subunit. The GABAB receptor
increases K+ conductance, thus generating an IPSP, and is activated by the agonist baclofen. GABA receptors are highly prevalent throughout the GNS (Kandel, p. 219)
- What ion blocks the ion pore of the iV-methyl-D-aspartate (NMDA) glutamate receptor at resting membrane potential?
A. Ca2+
B. Na+
C. K+
D. Mg2+
E. cr
D. Mg2+
Glutamate receptors are ionotropic channels that
induce EPSPs and include the Ar-methyl-D-aspartate glu-tamate (NMDA) receptor, the kainate receptor, and the kainate-quisqualate (AMPA) receptor. The NMDA receptor is an ion channel of high conductance that is permeable to Ca2+ , Na+, and K+ . This receptor contributes only to the later phases of the EPSP because it is active only in the presence of its ligand and membrane depolarization. This channel is unique in this regard because its activity is dependent upon a neurotransmitter and membrane potential. At resting membrane potential, Mg2+ blocks the ion pore of the NMDA channel. With membrane depolarization, the Mg2+ is dis-placed from the channel, allowing ion conduction to occur efficiently. The opening of the NMDA channel also requires glycine as a cofactor. The NMDA channel is inhibited by PGP and selectively blocked by APV. This channel is important in long-term potentiation at the neuronal synapse because its activity leads to an increase in cytosolic Ga2+ with sub-sequent activation of second messengers involved with long-lasting synaptic modifications. High levels of glutamate are toxic to neurons, and this toxicity is mediated primarily
by the NMDA channel. With excessive levels of glutamate, cytosolic Ca2+ increases significantly, activating proteases and phospholipases that generate free radicals toxic to neurons. The non-NMDA glutamate receptors (kainate and AMPA) are permeable to Na+ and K+ and are responsible for the generation of the early, large component of the EPSP. There are also metabotropic glutamate receptors that act through G proteins and second-messenger systems (Kandel,pp. 219-221)
- Inhibits glycine release
A. Tetrabenazine
B. a-bungarotoxin
C. D-tubocurarine
D. Strychnine
E. Tetanus toxin
F. Cholera toxin
G. Barbiturates
H. Botulinus toxin
I. Pertussis toxin
J. LSD
K. Ondansetron
L. None of the above
E. Tetanus toxin
Ion channels conduct ions at extremely high rates, are selective for specific ions, and are regulated or gated. Gated ion channels can be regulated by changes in voltage, chemical transmitters (ligands), and mechanical factors. Ligand-gated channels include glutamatergic channels, cholinergic channels, glycinergic channels, and GABAergic channels.
Acetylcholine-activated channels include nicotinic and
muscarinic receptors. Nicotinic cholinergic receptors are ionotropic channels that are permeable to Na+
and K+ and consist of five subunits: two a and the (3, y, and 8 subunits (a2py8). The a subunit binds a single molecule of ACh, thus requiring two ACh molecules to bind the receptor to elicit channel activation. The snake venom toxin cc-bungarotoxin binds the a subunit as well, effectively inhibiting channel function. Each subunit of the receptor contains four hydro-phobic a helices (Ml to M4) that traverse the plasma mem-brane. Opening of the nicotinic ACh channel results in the generation of a fast excitatory postsynaptic potential (EPSP).
Hexamethonium (ganglionic), succinylcholine (depolarizing), and D-tubocurarine (nondepolarizing) represent inhibitors of various nicotinic cholinergic receptors. The nicotinic receptor is found at the neuromuscular junction as well as preganglionic synapses of the autonomic nervous system.
The muscarinic cholinergic receptor is a metabotropic
receptor that is coupled to G proteins and consists of only two subunits (a and (3). This channel is a slow-activating K+ channel (M-type channel) that closes when stimulated and results in the generation of a slow EPSP. Muscarinic channels are found throughout the GNS (cerebellum, striatum, cortex,
Renshaw cells of the spinal cord) and in autonomic ganglia. These receptors are inhibited by atropine and scopolamine and stimulated by bethanecol (bladder), carbachol (Gl tract), pilocarpine (eye), and methacholine. Glycine is the neurotransmitter released by Renshaw cells (inhibitory interneurons) of the spinal cord (see dis-cussion, question 45). Glycine channels are blocked by strychnine, and glycine release is inhibited by tetanus toxin. There are five major groups of dopamine and serotonin receptors, all of which are metabotropic. Examples include Dl and D2 receptors, which stimulate and inhibit adenylyl cyclase, respectively. The net effect of Dl receptors is hyper-polarization, while that of D2 receptors is depolarization. The typical antipsychotics selectively inhibit D2 receptors. The majority of serotonin receptors are metabotropic. LSD is an agonist of the 5-HT 1G receptor, while ondansetron is
an antagonist of the 5-HT3 (ionotropic) receptor. All nora-drenergic receptors are metabotropic receptors that utilize G proteins and the second messenger cAMP. The signal transduction cascade involved in metabotropic receptor activation can be manipulated by cholera toxin, which selectively activates Gs, and pertussis toxin which inactivates Gj. Tetanus toxin specifically cleaves the protein synaptobrevin, while botulinus toxins cleave t-SNAREs and v-SNAREs, which subsequently results in the inhibition of
synaptic vesicle release at the terminal. The docking, fusion, and release of synaptic vesicles appears to involve distinct interactions between vesicle proteins (synaptobrevin and synaptotagmin, v-SNAREs) and proteins of the nerve terminal plasma membrane (syntaxins and neurexins, t-SNAREs) (Kandel, pp. 196-200, 219, 241-243, 1197-1199,1215-1216).
- Binds to a subunit of nicotinic receptors
A. Tetrabenazine
B. a-bungarotoxin
C. D-tubocurarine
D. Strychnine
E. Tetanus toxin
F. Cholera toxin
G. Barbiturates
H. Botulinus toxin
I. Pertussis toxin
J. LSD
K. Ondansetron
L. None of the above
B. a-bungarotoxin
Ion channels conduct ions at extremely high rates, are selective for specific ions, and are regulated or gated. Gated ion channels can be regulated by changes in voltage, chemical transmitters (ligands), and mechanical factors. Ligand-gated channels include glutamatergic channels, cholinergic channels, glycinergic channels, and GABAergic channels.
Acetylcholine-activated channels include nicotinic and
muscarinic receptors. Nicotinic cholinergic receptors are ionotropic channels that are permeable to Na+
and K+ and consist of five subunits: two a and the (3, y, and 8 subunits (a2py8). The a subunit binds a single molecule of ACh, thus requiring two ACh molecules to bind the receptor to elicit channel activation. The snake venom toxin cc-bungarotoxin binds the a subunit as well, effectively inhibiting channel function. Each subunit of the receptor contains four hydro-phobic a helices (Ml to M4) that traverse the plasma mem-brane. Opening of the nicotinic ACh channel results in the generation of a fast excitatory postsynaptic potential (EPSP).
Hexamethonium (ganglionic), succinylcholine (depolarizing), and D-tubocurarine (nondepolarizing) represent inhibitors of various nicotinic cholinergic receptors. The nicotinic receptor is found at the neuromuscular junction as well as preganglionic synapses of the autonomic nervous system.
The muscarinic cholinergic receptor is a metabotropic
receptor that is coupled to G proteins and consists of only two subunits (a and (3). This channel is a slow-activating K+ channel (M-type channel) that closes when stimulated and results in the generation of a slow EPSP. Muscarinic channels are found throughout the GNS (cerebellum, striatum, cortex,
Renshaw cells of the spinal cord) and in autonomic ganglia. These receptors are inhibited by atropine and scopolamine and stimulated by bethanecol (bladder), carbachol (Gl tract), pilocarpine (eye), and methacholine. Glycine is the neurotransmitter released by Renshaw cells (inhibitory interneurons) of the spinal cord (see dis-cussion, question 45). Glycine channels are blocked by strychnine, and glycine release is inhibited by tetanus toxin. There are five major groups of dopamine and serotonin receptors, all of which are metabotropic. Examples include Dl and D2 receptors, which stimulate and inhibit adenylyl cyclase, respectively. The net effect of Dl receptors is hyper-polarization, while that of D2 receptors is depolarization. The typical antipsychotics selectively inhibit D2 receptors. The majority of serotonin receptors are metabotropic. LSD is an agonist of the 5-HT 1G receptor, while ondansetron is
an antagonist of the 5-HT3 (ionotropic) receptor. All nora-drenergic receptors are metabotropic receptors that utilize G proteins and the second messenger cAMP. The signal transduction cascade involved in metabotropic receptor activation can be manipulated by cholera toxin, which selectively activates Gs, and pertussis toxin which inactivates Gj. Tetanus toxin specifically cleaves the protein synaptobrevin, while botulinus toxins cleave t-SNAREs and v-SNAREs, which subsequently results in the inhibition of
synaptic vesicle release at the terminal. The docking, fusion, and release of synaptic vesicles appears to involve distinct interactions between vesicle proteins (synaptobrevin and synaptotagmin, v-SNAREs) and proteins of the nerve terminal plasma membrane (syntaxins and neurexins, t-SNAREs) (Kandel, pp. 196-200, 219, 241-243, 1197-1199,1215-1216).
- Cleaves the protein synaptobrevin
A. Tetrabenazine
B. a-bungarotoxin
C. D-tubocurarine
D. Strychnine
E. Tetanus toxin
F. Cholera toxin
G. Barbiturates
H. Botulinus toxin
I. Pertussis toxin
J. LSD
K. Ondansetron
L. None of the above
E. Tetanus toxin
Ion channels conduct ions at extremely high rates, are selective for specific ions, and are regulated or gated. Gated ion channels can be regulated by changes in voltage, chemical transmitters (ligands), and mechanical factors. Ligand-gated channels include glutamatergic channels, cholinergic channels, glycinergic channels, and GABAergic channels.
Acetylcholine-activated channels include nicotinic and
muscarinic receptors. Nicotinic cholinergic receptors are ionotropic channels that are permeable to Na+
and K+ and consist of five subunits: two a and the (3, y, and 8 subunits (a2py8). The a subunit binds a single molecule of ACh, thus requiring two ACh molecules to bind the receptor to elicit channel activation. The snake venom toxin cc-bungarotoxin binds the a subunit as well, effectively inhibiting channel function. Each subunit of the receptor contains four hydro-phobic a helices (Ml to M4) that traverse the plasma mem-brane. Opening of the nicotinic ACh channel results in the generation of a fast excitatory postsynaptic potential (EPSP).
Hexamethonium (ganglionic), succinylcholine (depolarizing), and D-tubocurarine (nondepolarizing) represent inhibitors of various nicotinic cholinergic receptors. The nicotinic receptor is found at the neuromuscular junction as well as preganglionic synapses of the autonomic nervous system.
The muscarinic cholinergic receptor is a metabotropic
receptor that is coupled to G proteins and consists of only two subunits (a and (3). This channel is a slow-activating K+ channel (M-type channel) that closes when stimulated and results in the generation of a slow EPSP. Muscarinic channels are found throughout the GNS (cerebellum, striatum, cortex,
Renshaw cells of the spinal cord) and in autonomic ganglia. These receptors are inhibited by atropine and scopolamine and stimulated by bethanecol (bladder), carbachol (Gl tract), pilocarpine (eye), and methacholine. Glycine is the neurotransmitter released by Renshaw cells (inhibitory interneurons) of the spinal cord (see dis-cussion, question 45). Glycine channels are blocked by strychnine, and glycine release is inhibited by tetanus toxin. There are five major groups of dopamine and serotonin receptors, all of which are metabotropic. Examples include Dl and D2 receptors, which stimulate and inhibit adenylyl cyclase, respectively. The net effect of Dl receptors is hyper-polarization, while that of D2 receptors is depolarization. The typical antipsychotics selectively inhibit D2 receptors. The majority of serotonin receptors are metabotropic. LSD is an agonist of the 5-HT 1G receptor, while ondansetron is
an antagonist of the 5-HT3 (ionotropic) receptor. All nora-drenergic receptors are metabotropic receptors that utilize G proteins and the second messenger cAMP. The signal transduction cascade involved in metabotropic receptor activation can be manipulated by cholera toxin, which selectively activates Gs, and pertussis toxin which inactivates Gj. Tetanus toxin specifically cleaves the protein synaptobrevin, while botulinus toxins cleave t-SNAREs and v-SNAREs, which subsequently results in the inhibition of
synaptic vesicle release at the terminal. The docking, fusion, and release of synaptic vesicles appears to involve distinct interactions between vesicle proteins (synaptobrevin and synaptotagmin, v-SNAREs) and proteins of the nerve terminal plasma membrane (syntaxins and neurexins, t-SNAREs) (Kandel, pp. 196-200, 219, 241-243, 1197-1199,1215-1216).
- Cleave t-SNAREs and v-SNAREs
A. Tetrabenazine
B. a-bungarotoxin
C. D-tubocurarine
D. Strychnine
E. Tetanus toxin
F. Cholera toxin
G. Barbiturates
H. Botulinus toxin
I. Pertussis toxin
J. LSD
K. Ondansetron
L. None of the above
H. Botulinus toxin
Ion channels conduct ions at extremely high rates, are selective for specific ions, and are regulated or gated. Gated ion channels can be regulated by changes in voltage, chemical transmitters (ligands), and mechanical factors. Ligand-gated channels include glutamatergic channels, cholinergic channels, glycinergic channels, and GABAergic channels.
Acetylcholine-activated channels include nicotinic and
muscarinic receptors. Nicotinic cholinergic receptors are ionotropic channels that are permeable to Na+
and K+ and consist of five subunits: two a and the (3, y, and 8 subunits (a2py8). The a subunit binds a single molecule of ACh, thus requiring two ACh molecules to bind the receptor to elicit channel activation. The snake venom toxin cc-bungarotoxin binds the a subunit as well, effectively inhibiting channel function. Each subunit of the receptor contains four hydro-phobic a helices (Ml to M4) that traverse the plasma mem-brane. Opening of the nicotinic ACh channel results in the generation of a fast excitatory postsynaptic potential (EPSP).
Hexamethonium (ganglionic), succinylcholine (depolarizing), and D-tubocurarine (nondepolarizing) represent inhibitors of various nicotinic cholinergic receptors. The nicotinic receptor is found at the neuromuscular junction as well as preganglionic synapses of the autonomic nervous system.
The muscarinic cholinergic receptor is a metabotropic
receptor that is coupled to G proteins and consists of only two subunits (a and (3). This channel is a slow-activating K+ channel (M-type channel) that closes when stimulated and results in the generation of a slow EPSP. Muscarinic channels are found throughout the GNS (cerebellum, striatum, cortex,
Renshaw cells of the spinal cord) and in autonomic ganglia. These receptors are inhibited by atropine and scopolamine and stimulated by bethanecol (bladder), carbachol (Gl tract), pilocarpine (eye), and methacholine. Glycine is the neurotransmitter released by Renshaw cells (inhibitory interneurons) of the spinal cord (see dis-cussion, question 45). Glycine channels are blocked by strychnine, and glycine release is inhibited by tetanus toxin. There are five major groups of dopamine and serotonin receptors, all of which are metabotropic. Examples include Dl and D2 receptors, which stimulate and inhibit adenylyl cyclase, respectively. The net effect of Dl receptors is hyper-polarization, while that of D2 receptors is depolarization. The typical antipsychotics selectively inhibit D2 receptors. The majority of serotonin receptors are metabotropic. LSD is an agonist of the 5-HT 1G receptor, while ondansetron is
an antagonist of the 5-HT3 (ionotropic) receptor. All nora-drenergic receptors are metabotropic receptors that utilize G proteins and the second messenger cAMP. The signal transduction cascade involved in metabotropic receptor activation can be manipulated by cholera toxin, which selectively activates Gs, and pertussis toxin which inactivates Gj. Tetanus toxin specifically cleaves the protein synaptobrevin, while botulinus toxins cleave t-SNAREs and v-SNAREs, which subsequently results in the inhibition of
synaptic vesicle release at the terminal. The docking, fusion, and release of synaptic vesicles appears to involve distinct interactions between vesicle proteins (synaptobrevin and synaptotagmin, v-SNAREs) and proteins of the nerve terminal plasma membrane (syntaxins and neurexins, t-SNAREs) (Kandel, pp. 196-200, 219, 241-243, 1197-1199,1215-1216).


