Neoplasia 3 Flashcards
What is the differential diagnosis of a mass lesion?
- neoplastic or non-neoplastic (abscess, etc.)?
- if neoplastic:
- benign or malignant?
- what type is it? epithelial, mesenchymal, etc.
- if malignant:
- primary or metastatic?
How might a tumour present clinically?
local effects of primary tumours:
- eg lung - cough, haemoptysis, wheeze, dyspnoea, pneumonia, Pancoasts’s syndrome (apical tumours)
effects of metastases:
- local lymphadenopathy
- bone pain or features related to hypercalcaemia
- jaundice
- seizures (metastasis in brain)
- cachexia (larger tumours)
- weight loss induced by TNFa and IL-1 produced by the tumour cells or stroma that increases the basal metabolic rate, therefore using up more nutrients
paraneoplastic syndromes
*most is caught by screening prior to these presentations
What are paraneoplastic syndromes, and how do they develop?
- effects of cancer not due to the mass/lesion or hormones produced by them
- can be endocrine:
- caused by hormones produced by cell types that don’t normally produce them
- e.g. tumours produce ADH or ACTH (Cushings) inappropriately (small cell carcinoma)
- hypercalcemia from PTHrP (SCC)
- caused by hormones produced by cell types that don’t normally produce them
- can be immunologic
- dermatologic (rashes and muscle syndromes), neural (small cell carcinoma)
- nephrotic syndrome
- others:
- clubbing and hypertrophic osteoarthropathy (lung cancer)
- vascular and haemotologic effects like DVT, non-bacterial thrombotic endocarditis
What is cancer cachexia?
- weight loss induced by TNFa and IL-1 produced by tumour cells or tumour stroma increasing the basal metabolic rate and tf use of nutrients
How are neoplastic lesions investigated and diagnosed pathologically?
- clinical history and exam
- FBE
- liver function, tumour markers (prostate specific antigen, carcinoembryonic antigen, alpha fetoprotein)
- radiology to investigate spread
- XR, CT, US
- endoscopy/bronchoscopy (lung cancer)
- tissue sampling/biopsy
- pathological dx to confirm malignancy, determine features important in prognosis and management
What is the difference between cytological and histopathological diagnosis?
- cytology
- fine needle aspiration of tumour cells (not tissue)
- sputum
- bronchial washings
- histopathology on larger samples of tissue
- stained (H&E, special) and immunohistochem to determine cell lineage
- molecular and cytogenic techniques:
- in-situ hybridization, PCR, chromosomal rearrangements
- others e.g. flow cytometery

What are tumour markers?
- measured in blood, produced in tumour cells
- not usually used in diagnosis because they are not specific
- usually used in follow up to assess tumour regrowth or reocurrence
- eg
- prostate specific antigen
- carcinoembryonic antigen (CEA)
- alpha fetoprotein
*
What is definitive diagnosis of malignancy based on?
- recognition of particular cytologic and architectural features
- cellular features indicating the cell lineage of the tumour
- grade
- stage
- presence of lymphovascular invasion
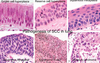
How is immunohistochemistry used to diagnose and assess neoplastic lesions?
- when tumour type cannot be determined on H&E or special staining
- using protein Abs with immunofluorescent, enzyme, or peroxidase label
- basing cell type on phenotypic expression of different proteins
- enzyme changes the substrate, the label changes colour
- when it binds to it’s target protein there is a colour change
- examples:
- protein S100 - on melanocytes; stain brown if present = melanoma
- CAM5.2 - protein in epithelium; stain brown if present = epithelial cells, carcinoma
- Leukocyte common antigen (CD45) - lymphoid marker; leukocytes in tumours will stain brown (bottom L)

What techniques are used to diagnose and assess neoplastic lesions?
- H&E
- light microscopy
- immunohistochemistry
What factors influence the behaviour of tumours and their prognosis?
- type
- grade
- stage
- vascular invasion seen in primary tumour
- may not be clinical evidence of metastases yet
- worse prognosis if this is seen histologically
- genetic alterations may influence prognosis and tx
- in some tumours, ulceration and patterns of inflammation influence prognosis
- predictive factors that predict likely response to certain therapies
- eg HER2 amplification, estrogen and progeseterone receptors in breast cancer
What factors influence the degree of differentiation (grade) and stage of a tumour ?
Grade/Differentiation
- well differentiated tumours tend to be benign, whereas poorly differentiated tumours tend to be malignant
- determined pathologically or by scales (Gleason score in carcinoma of prostate, Modified Bloom and Richardson in carcinoma of breast)
Stage
- stage refers to the progression the malignancy has made in terms of local spread and metastasis
- incorporates the size or depth of invasion, local extent of primary tumour, and location and extent of metastases
- determined radiologically and pathologically
- most staging systems have 4 stages
- any tumour with distant metastasis is stage 4
- eg TMN for lung cancer, stage depends on T0-4 (primary tumour) and N0-3(lymph nodes), if metastasis (M), it’s stage 4
What are the principles of management of malignancy?
- surgery
- specimen –> pathology for report on prognostic information etc:
- confirmation/further confirmation on type and subtype (lineage) of malignancy
- grade
- size of tumour/depth of invasion
- +/- microscopic vascular invasion
- completeness of surgical excision
- presence and number of lymph node metastases (may only be microscopic, requires histological examination)
- specimen –> pathology for report on prognostic information etc:
- radiotherapy
- chemotherapy
- targeted therapy
- immunotherapy to enhance immune response (eg melanoma)
- bone marrow transplant
What is the nature and role of targeted therapies in malignancy?
- traditional chemo drugs interfere with cell division and tf of normal cells
- targeted therapies block growth of cancer cells
- interfere with specific molecules (oncoproteins) that drive carcinogenesis and tumour growth
- less harmful, aims to target cancer cells
- eg stop angiogenisis w/VGEF inhibitors; stop growth of stroma with TGFbeta inhibitors
- not all pt with the same tumour have same phenotype and genotype, tf must test tumours to assess responsiveness (eg HER2 amplification in breast cancer)
- two main categories:
- small molecules that eg inhibit GF receptors or tyrosine kinase
- monoclonal Abs that target specific proteins or receptors
How can common cancers be prevented?
- public education campaigns
- personal measures
- healthy diet
- exercise
- not smoking
- screening programs
- laws regulating exposure, safety measures in the workplace