Molecular Shape Blurb Flashcards
Describe the shape and angle of a molecule from the lewis diagram
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the C central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
4 are bonding and 0 non-bonding
Tetrahedral
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the As central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
3 are bonding and 1 non-bonding
Trigonal pyramid
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the central O atom.
4 electron areas is tetrahedral, bond angle of 109.5o
2 are bonding and 2 non-bonding
bent
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…
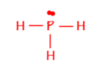
minimising repulsion of the 4 electron areas around the P central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
3 are bonding and 1 non-bonding
Trigonal pyramid
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the N central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
3 are bonding and 1 non-bonding
Trigonal pyramid
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the central O atom.
4 electron areas is tetrahedral, bond angle of 109.5o
2 are bonding and 2 non-bonding
bent
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the C central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
4 are bonding and 0 non-bonding
Tetrahedral
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 2 electron areas around the C central atom.
2 electron areas is linear, bond angle of 180o
2 are bonding and 0 non-bonding
Linear
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the P central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
3 are bonding and 1 non-bonding
Trigonal pyramid
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…
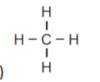
minimising repulsion of the 4 electron areas around the C central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
4 are bonding and 0 non-bonding
Tetrahedral
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…
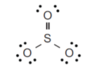
minimising repulsion of the 3 electron areas around the S central atom.
3 electron areas is trigonal planar, bond angle of 120o
3 are bonding and 0 non-bonding
Trigonal planar
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…
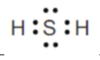
minimising repulsion of the 4 electron areas around the central O atom.
4 electron areas is tetrahedral, bond angle of 109.5o
2 are bonding and 2 non-bonding
bent
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 2 electron areas around the N central atom.
2 electron areas is linear, bond angle of 180o
2 are bonding and 0 non-bonding
Linear
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…
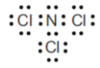
minimising repulsion of the 4 electron areas around the N central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
3 are bonding and 1 non-bonding
Trigonal pyramid
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the C central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
4 are bonding and 0 non-bonding
Tetrahedral
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 2 electron areas around the C central atom.
2 electron areas is linear, bond angle of 180o
2 are bonding and 0 non-bonding
Linear
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 3 electron areas around the S central atom.
3 electron areas is trigonal planar, bond angle of 120o
2 are bonding and 1 non-bonding
Bent
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the P central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
3 are bonding and 1 non-bonding
Trigonal pyramid
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the central S atom.
4 electron areas is tetrahedral, bond angle of 109.5o
2 are bonding and 2 non-bonding
bent
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…
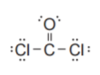
Trigonal planar, 120o
Polar, symmetric, different bonds
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 4 electron areas around the C central atom.
4 electron areas is tetrahedral, bond angle of 109.5o
4 are bonding and 0 non-bonding
Tetrahedral
Shape and angle are determined by…
The shape that minimises repulsion for…
As… (bonding vs non-bonding)
The shape is…

minimising repulsion of the 3 electron areas around the C central atom.
3 electron areas is trigonal planar, bond angle of 120o
3 are bonding and 0 non-bonding
Trigonal planar


