Module 1: DFT Flashcards
1
Q
Main Challenges for DFT
A
- dispersion forces
- due to long-range correlations between zero-point fluctuations of dipole moments
- (semi-)local functionals cannot capture this long-range interactions
- overbinding
- usually leads to higher energies, shorter bond lengths, etc.
- self-interaction
2
Q
Dispersion Correction
A
- correct for dispersion by adding dispersion energy to Kohn-Sham energy
- different methods
- DFTD3
- TS-vdW
- vdW-DF
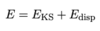
3
Q
Dispersion Energy
A
- Dispersion energy determined by dispersion coefficients Cx and separation distance rx
- C6 ≡ vdW/London dispersion
- calculated analytically using Casimir-Polder formula

4
Q
DFTD3
A
- uses C6, C8 terms to calculate dispersion energy
- use AmHn/BkHl referece hydrides to compute molecular polarizabilities α(iω) and subtract contribution due to H2 component
- Requires TDDFT calculations
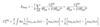
5
Q
TS-vdW
A
- semi-empirical
- Calculate effective C6,AB using geometric-like mixing of effective same-species C6,ii weighted by static polarizabilities
- Effective C6,ii modified by Hirschfield colume partitioning function

6
Q
Local Density Approximation
(Overview)
A
- uses per electron exchange-correlation energy of homogeneous electron gas
- exchange known analytically
- correlation known analytically or numerically exact
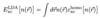
7
Q
Local Density Approximation
(Good Performance)
A
- structural, elastic, and vibrational properties
- material science
8
Q
Local Density Approximation
(Bad Performance)
A
- overbinds → binding energies too high
- underestimates lattice constants
- unreliable activation energies for chemical reactions
- energetics of magnetic materials
9
Q
Generalized Gradient Approximation
(Overview)
A
- enhancement factor Fxc over LDA
- contains next term in derivative expansion of density
- no universal form
- e.g. PBE (Perdew-Burke-Ernzerhof)
- more repulsive core-valence xc → increase in bulk lattice contants
- reduced valence effects → decrease in cohesive energies

10
Q
Generalized Gradient Approximation
(Good Performance)
A
- atomic and molecular total energies improved
- corrects LDA overbinding
- improved activation energies (still too low, though)
- more relaistic magnetic solids
11
Q
Generalized Gradient Approximation
(Bad Performance)
A
- softened bonds → increase lattice constants
- dispersion not included
12
Q
Exchange Correlation Exact Definition
A
- Coulomb interaction between electron at r and value of xc-hole nxc(r,r’) at r’
- nxc not known exactly

13
Q
Hybrid Functionals
A
e.g. B3LYP

14
Q
vdW-DF
A
- adds non-local term with kernal function

15
Q
TDDFT
A
- uses Runge-Gross Theorem for quantum mechanical action to define xc energy
- requires new functionals that include (non-)locality in space and time


