Metabolism & Cell Respiration (//unit 8) Flashcards
definition of cell respiration
The set of metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products.
Activation energy, catalysis, transition state, and exothermic reactions
– During chemical reactions, reactants are converted into products. Before a molecule can take part in the reaction, it needs to gain activation energy, which breaks bonds within the reactant.
– Later during the reaction, energy is given out as new bonds are made. Most biological reactions are exothermic - the energy released is greater than the activation energy.
– Enzymes reduce the activation energy via catalysis. The active site of an enzyme causes changes within the substrate molecule, which weakens its bonds. It is changed into a transition state, which has less energy and, thereby, makes reactions easier to occur. The conformation of the protein is altered and the shape of the active site becomes complementary to that of the substrate. After that, the active site returns to its original conformation.
Calculating rates of reaction
Can be assed by measuring the quantity of substrate used per unit time or of a product formed per unit time. These quantities can be mass or volume (e.g. decreasing mass in potato lab).
competitive and non-competitive inhibitors
Enzyme inhibitors are chemical substances that reduce or prevent the activity of enzymes.
Competitive inhibitor:
- the substrate and inhibitor are chemically very similar
- the inhibitor binds to the active site
- while the inhibitor occupies the active site, it prevents the substrates from binding, hence, residing the enzyme’s productivity
- As the substrate concentration rises, the effect of the inhibitor becomes less and less until eventually it is negligible
Non-competitive inhibitor:
- the substrate and inhibitor are inhibitor are not similar
- the inhibitor binds to the enzyme at a different site from the active site
- this changes the conformation of the enzyme’s, even when the substrate binds to the active site, it can not be catalysed and is only carried out very slowly
- The activity is reduced at all substrate concentrations if a fixed concentration of non-competitive inhibitor is added and the percentage reduction is the same at all substrate concentrations. This is because the inhibitor is not competing for the active site, in contrast to the competitive inhibitor, and, hence, affects the same proportion of enzymes at all substrate concentrations. The substrate cannot prevent the binding of the inhibitor as it binds somewhere else than the active site.

Features of metabolic pathways
& catabolic vs anabolic pathways
- an enzyme catalyses each reaction in the pathway
- all reactions occur inside cells
- produce products and waste products (plus intermediates sometimes)
- some pathways build up organic compounds (anabolic pathways) (e.g. insulin) and some break them down (catabolic pathways) (e.g. glycolysis; from one glucose to two triose phosphate)
- some metabolic pathways consists of chains of reactions (e.g. link reaction)
- some metabolic pathways consists of cycles of reactions, where a substrate is continually regenerated by the cycle (e.g. Krebs cycle)
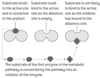
End-product inhibition and allosteric enzymes (whats the benefit?)
In many metabolic pathways, the product of the last reaction in the pathway inhibits the enzyme that catalyses the first reaction — end-product inhibition.
The enzyme that is inhibited by the end products is an example of an allosteric enzyme. Allosteric enzymes have two non-overlapping binding sites. One active site and one allosteric site. With end-product inhibition the allosteric site is a binding site for the end product. When it binds, the structure of the enzyme is altered so substrates less likely bind to the active site. Binding of the inhibitor is reversibel and if it detaches, the enzyme returns to its original conformation.
Negative feedback: The advantage of this method of controlling metabolic pathways is that if there is an excess of the end product the whole pathway is switched off and intermediates do not build up. Conversely, as the level of the end product falls, more and more of the enzymes that catalyse the first reaction will start to work and the whole pathway will become activated.
Finding new anti-malarial drugs
The malarial parasite (Plasmodium) constantly evolves resistances to drugs so there is an urgent need for new drugs. The search is made more effective by the large bioinformatics databases in computers. Possible enzyme inhibitors for Plasmodium are identified and researched.
oxidation and reduction
Oxdiation
- addition of oxygen
- removal of hydrogen and electrons (simultaneously)
Reduction
- removal of oxygen
- addition of hydrogen and electrons (simultaneously)
NAD + 2H (aka. 2 electrons) —> reduced NAD
phosphorylation
adding a phosphate group to a molecule
- makes it less stable and therefore more likely to react
- can turn an endothermic (endergonic) reaction that will only occur at a very slow rate into an exothermic (exergonic) reaction that can proceed rapidly.
The phosphate group is usually transferred from ATP.
Example: glucose + ATP —> glucose 6-phosphate + ADP
glycolysis (general description + stages)
If glucose is the substrate, the first stage of cell respiration is a metabolic pathway called glycolysis. The pathway is catalysed by enzymes in the cytoplasm.
Glucose is partially oxidised and a small amount of ATP is produced, which is the anaerobic respiration.
Four main stages:
- Two phosphate groups are added to a molecule of glucose to form hexose biphosphate — phosphorylation. Two ATP molecules provide the phosphate groups. The energy level of hexose is raised by phosphorylation, so it is less stable.
- The hexose biphosphate is split to form two molecules of triose phosphate — lysis.
- Two atoms of hydrogen are removed from each triose phosphate molecule — oxidation.
- The energy released by the oxidation of each triose phosphate molecule is used to convert two ADP molecules to ATP. The end product of glycolysis is pyruvate.
Summary
- one glucose is converted into two pyruvate
- two NADs are converted into two reduced NADs
- two ATP molecules are used per glucose but four are produced so there is a net yield of two ATP. This is a small yield of ATP per glucose.

Overview of link reaction and Krebs cycle
After pyruvate is produced in glycolysis, it can only be oxidised further with the release of more energy from oxygen. So when oxygen is available, the pyruvate is absorbed in the mitochondrion to be oxidised and decarboxylated — the link reaction.
Enzymes in the matrix of the mitochondrion the catalyse a cycle of reactions called the Krebs cycle. The removed hydrogens are accepted by NAD and FAD and passed on to the electron transport chain where oxidative phosphorylation occurs.
Link reaction
Pyruvate from glycolysis is absorbed by the mitochondrion. Enzymes in the matrix of the mitochondrion remove hydrogen (oxidation) and carbon dioxide (decarboxylation) from the pyruvate.
The hydrogen is accepted by NAD — oxidative decarboxylation. The product is acetyl coenzyme A.

Krebs cycle
Acetyl groups from the link reaction are fed into the Krebs cycle. In the first reaction of the cycle an acetyl group is transferred from acetyl CoA to a four-carbon compound (oxaloacetate) to form a six-carbon compound (citrate). Citrate is converted back into oxaloacetate in the following reactions of the cycle.
- Three types of reactions are involved per cycle:*
- Carbon dioxide is removed in two decarboxylation reactions.
- The carbon dioxide is a waste product and is excreted together with the carbon dioxide from the link reaction.
- Hydrogen is removed in four oxidation reactions.
- The hydrogen is accepted by hydrogen carriers, which become reduced.
- In three of the oxidations the hydrogen is accepted by NAD, in the other it’s FAD.
- These oxidation reactions release energy, much of which is stored by the carriers when they accept hydrogen. The energy is later released by the electron transport chain and used to make ATP.
- One ATP is produced directly in the substrate-level phosphorylation reaction.
Summary
In the Krebs cycle, the oxidation of acetyl groups is coupled with the reduction of hydrogen carriers. This chemical energy is then passed on to oxidative phosphorylation.
chemiosmosis - oxdidative phosphorylation
The electron transport chain is a series of electron carriers, located in the inner plasma membrane of the mitochondrion including the cristae. Reduced NAD supplies two electors to the first carrier in the chain. As the electrons pass along the chain from one carrier to the next they give up energy. Some of the electron carriers act as proton pumps and use this energy to pump protons (H+) against the concentration gradient from the matrix to the intermembrane space. This creates potential energy. Reduced FAD also feeds electrons in to the electron transport chain, but at a slightly later state than reduced NAD.
Chemiosmosis: ATP synthase allows the protons to diffuse down the concentration gradient back to the matrix and uses the energy of that passive movement through rotation, producing ATP.
Oxygen is the final electron acceptor, which happens in the matrix, on the surface of the inner membrane. As H+ fuses with O, water is created - H2O. This maintains the proton gradient. This is the only time where oxygen is used in cell respiration.
If oxygen is not available, electron flow along the electron transport chain stops and reduced NAD cannot be converted back to NAD. Supplies of NAD in the mitochondrion run out and the link reaction and Krebs cycle cannot continue. Only glycolysis continues with a small yield of ATP. Hence, oxygen greatly increases ATP yield, per glucose, of cell respiration.
Mitochondrion - structure and function
The close relation between structure and function is a reoccurring theme in biology and known as adaption, which is the result of evolution by natural selection.
Mitochondrial membranes are dynamic
Electron tomography can obtain three-dimension images of active mitochondria. It has revealed that cristae are connected with the intermembrane space between the inner and outer membranes via arrow openings. The shape and volume of the cristae change when a mitochondrion is active in ways that are still being investigated.
The mitochondrion is a semi-autonomous organelle in that it can grow and reproduce itself but it still depends on the rest of the cell for resources and is otherwise part of the cellular system. 70S ribosomes and a naked loop of DNA are found within the mitochondrial matrix.



