Lysosomes Flashcards
(16 cards)
What is the pH inside a lysosome?
They have a pH between 4-5.
Why is the pH acidic inside a lysosome?
- The hydrolytic enzymes need an acidic pH to be active.
How many lysosome are there in an average cell?
There are about 100 lysomses.
What is the lysosome membrane described as?
It is described as a single membrane.
What are hydrolytic enzymes? How many hydrolytic enzymes are found in lysosomes? Give some examples (name and function).
- Hydrolytic enzymes are used for the controlled intracellular digestion of macromolecules.
- There are about 40 hydrolytic enzymes in lysosomes.
E.g.:-
- Proteases - Degrade proteins.
- Lipase - Digest lipids.
- Glycosidases - Digest polysaccharides (sugars).
- Nucleases - Digest RNA or DNA.
- Phosphatases / Sulfatases - Digesr phosphate and sulfate groups.
How does membrane protect itself from being digested?
- The membrane has a lot of transmembrane proteins inserted into it.
- These proteins are heavily gycosylated (to form a glycoprotein) on the luminal (inner) side of the lysosome.
- So there are very large sugar structures attached to them that helps protect the actual bilayer from the enzymes inside the lumen of the lysosome.
How is an acidic pH maintained in the lysosome?
- The membrane contains a hydrogen ion pump which requires ATP.
- This pump actively pumps H+ ions into the lysosome, thereby maintaining its acidity.
How are extracellular substrates delivered to the lysosomes?
- Fluid-phase endocytosis of molecules and lipoproteins (includes receptor-mediated endocytosis).
- Phagocytosis of particles that are greater than 0.5 µm.
Lipoproteins meaning - are complex’s of lipids and proteins that carry fats through the blood stream.
What are the two ways intracellular substrates are delivered to lysosomes?
- Microautophagy is where the membrane of the lysosome invaginates.
- Macroautophagy involves much bigger pieces of the cell like chunks of cytosol or entire organelles that are wrapped in ER membrane, which then fuses with lysosomes.
- Selective transport of proteins across the lysosomal membrane
Microautophagy is the type of autophagic pathway which is mediated by direct lysosomal or vacuolar engulfment of the cytoplasmic cargo.
Autophagy is the natural, regulated mechanism of the cell that disassembles unnecessary or dysfunctional components.
Invagination: the action or process of being turned inside out or folded back on itself to form a cavity or pouch.
Macroautophagy is a process in which cellular contents are degraded by lysosomes or vacuoles and recycled.

How does LDL (Low density lipoprotein) get into the lysosome?
- The LDL binds to the LDL surface receptors
- The receptors aggregate to form coated pits which can invaginate and then pinch off to form a vesicle.
- At this stage the pH starts to drop causing the LDL particle to dissociate from the receptor allowing the receptor to be recycled to vesicular transport so they can be reused again.

What are the cholesterol esters in the LDL hydrolysed into?
Cholesteryl ester and fatty acid by lipase enzymes.
How does phagocytosis occur?
- The plasma membrane wraps around the particle (engulfment). This forms a phagosome.
- The lysosome fuses with the phagosome and the pH drops causing digestion of the particle.
- The left over peptides, amino acids, sugars can be reused or recycled by the cell.

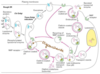
Describe the mannose 6-phosphate pathway.
The mannose 6-phosphate pathway is the major route for targeting lysosomal enzymes to lysosomes.
- Digestive enzymes and membrane proteins of lysosomes are synthesised in the rough ER and transported through the Golgi apparatus to the trans-Golgi network.
- In the Golgi apparatus the lysosomal enzyme (in pink) and secretory proteins (in blue) might both receive oligosaccharides (the tail).
- But the blue proteins end up at the plasma membrane and the pink proteins go to lysosomes.
- This happens because only the lysosomal enzymes acquire a modification called mannose 6-phosphate.
- This is used as a tag because once the proteins reach the trans Golgi the mannose 6-phosphate is recognised by a protein in the trans Golgi membrane called the mannose 6-phosphate receptor (M6P receptor) that capture the proteins.
- The M6P receptor binds to the M6P on the oligosaccharide of the lysosomal enzymes and captures the used proteins and diverts them from the secretory pathway and directs the proteins for transport to late endosomes and lysosomes.
- A clathrin coated vesicle (made of proteins) is formed that helps deform the membrane. They contain M6P receptor with its protein cargo.
- The clathrin coated vesicle breaks off into clathrin triskelions and the receptor comes off and the vesicle can bind with a lysosome.
- The vesicles are transported to late endosomes and then to lysosomes.
- The proteins that do not have M6P modification enter a different route of vesicles that also form at the trans Golgi but they take the default pathway and are transported to the plasma membrane.
- In some cases some lysosomal enzymes do escape through the default pathway. But if that happens there are M6P receptor molecules present at the plasma membrane that can capture any lysosomal enzymes that have been accidentally transported outside the cell.
- The route is in the complex being collected in the vesicles are ultimately transferred to late endosomes and then they end up at lysosomes.
[Clathrin is a complex of proteins that assembles on the membrane and that helps form the vesicle. It forms a lattice on the patch of membrane by interacting indirectly with M6P receptor. As it polymerises it deforms the membrane so that a vesicle can be formed.]
What is I-cell? What is it caused due to?
- I-cell disease is part of the lysosomal storage disease family and results from a defective phosphotransferase (an enzyme of the Golgi apparatus).
- Phosphotransferase is responsible for the attachment of the M6P residue to the proteins.
- These missing enzymes will cause no proteins to receive the M6P receptor so the proteins will behave like the blue proteins that are secreted from the cell.

What are some of the symptoms of I-cell disease?
- Skeletal abnormalities
- Developmental delay
- Enlarged liver and spleen
- Impaired hearing
- Death from pneumonia or congestive heart failure usually occurs within the first decade of life


