Lecture 10: Active Membrane Transport Flashcards
(55 cards)
Glucose Transporters
Rate of erythrocyte Glucose Transporter GLUT1
- Glucose enters the erythrocyte by facilitated diffusion via a specific glucose transporter
- rate 50,000 time greater than uncatalyzed membrane diffusion
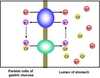
General structure of GLUT1
- 12 hydrophpbic segments
- each beleived to form a membrane spanning helix
Model of GLUT1
- side by side assemply of several helices produces a transmembrane corridor lined with hydrophilic residues that can hydrogen bond with a glucose as it movves through
Ser, Leu, Val, Thr, Asn, Phe, Ile
4 steps of GLUT1 trasnport
4 steps:
- binding
- transport
- dissociation
- recovery
Similarities of GLUT1 to enzymatic reaction
substrate is glucose outside of cell
product is glucose inside cell
enzyme is the trasnporter
plot of glucose uptake as a function of external glucose concentration
hyperbolic (like enzymes)
lineweaver burke
1/V0 = Km / (Vmax x [S]out) + 1/Vmax
GLUT2
- in epitherlial cells
- Na+ glucose symporter and glucose uniporter (GLUT2) opporate on opposite sides of epitherlial cells
- transporter systems located asymmetrically, each confined to one sid eof plasma membrane
GLUT4
- mainly in skeletal and heart muscle as well as adipose tissue
- can be distinguished from other glucose transporters by its response to insulin
- Activity increases when insulin signals a high blood glucose concentration, thus increasing the rate of glucose uptake into muscle and adipose tissue (15 fold or more)
Diagram of GLUT4
Comparison of GLUT transporters to Chloride Bicarbonate exchangers
GLUTs are uniport
Chloride bicarbonate are antiporters –> for each HCO3- ion that moves in one direction, one Cl- ion moves in the opposite
chloride bicarbonate exchangers
- for each HCO3- that moves in one direction, one Cl- ion moves in the opposite
- coupling is required for transport activity
- no net transfer of charge and exchange is electroneutral
- humanms have 3 AE isoforms, AE1 is predominant in erythrocytes
AE1 transporter
- in erythrocytes
- important CO2 trasnport and buffering blood pH
CO2 in blood
- CO2 that is produced by cellular respiration enters the blood and erythrocytes (RBCs) where it is quickly converted to bicarbonate ions and protons in a reaction catalyzed by carbonic anhydrase
- carbonic anhydrase catalyzes the formation of carbonic acid from carbon dioxide in water
Importance of carbonic anhydrase
- protons produced by the carbonic anhydrase reaction induce the Bohr shift, which makes the hemoglobin more likely to release the oxygen
- PCO2 in blood drops when CO2 is converted to bicarbonate, maintaining a strong partial pressure gradient favoring the entry of CO2 into red blood cells
* carbonic anhydrase activity makes CO2 uptake from tissues more efficient
Bohr Shift and oxygen release by hemoglobin
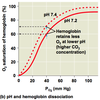
MEchanism of CO2 transport
- H+ produced by dissociation of carbonic acid is taken up by hemoglobin (hemoglobin also acts as a buffer, minimizing changes in pH)
- in the alveoli, a partial pressure gradient favors the diffusion of CO2 from plasma RBCs to the atmosphere
- hemoglobin releases protons, which combine wwith bicarbonate to form CO2, which then diffuses into the alveoli and is exhaled
- hemoglobin picks up O2 in inhalation and the cycle begins again
Important aspects of active transport
- results in accumulation of a solute above the equilibrium point
- thermodynamically unfavorable (endergonic)
- takes place only when coupled to exergonic process
- ie: coupled trasnport, ATP hydrolysis
Types of active trasnport
primary: uses ATP
Secondary: uses coupled trasnport
Primary active transport
- solute accumulation is coupled directly to an exergonic reaction, such as the hydrolysis of ATP
- P-type, V-type, F-type ATPases –> act like uniport or antiport pumps
- ABC (ATP binding cassette) trasnporters –> acts as uniporter
Secondary Active transport
- energy comes from an ion concentration gradient that is established by primary active trasnport
- sodium-solute or proton-solute contrasnporters, which are symporters or antiporters
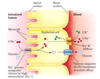
Energy dependent pumps
- two principal conformational states
- each open to one side of the membrane
- to pump a molecule (such as an ion) in a single direction across a membrane, the invested energy (eg. free energy of ATP hydrolysis) must be coupled to the interconversion between these conformational states
Diagram of energy dependent pumps
P Type ATPases
cation trasnporters that are reversibly phosphorylated by ATP as part of the transport cycle
–> phosphorylation forces are a conformational change that is central to movement of the cation
–> they are sensitive to inhibition by vandate and can act as uniporters (eg Ca2+ ATPases) or antiporters (eg Na+K+ ATPases)