Lect3: Intracellular protein sorting and vesicular transport Flashcards
(18 cards)
Two theories on compartment formation:
1: Invagination of cell membrane started by proteins which were the precursors to endocytotic vessel forming proteins (proteins like clathrin and caveolae)
2: Endosymbiote hypothesis for mitochondria
Cell composition:
- Relative ratio of hepatocytes by volume:
- Percentage of total cell membrane
- Packing:
- Relative ratio of hepatocytes by volume: 54% cytoplasm, 24% mitochondria, 9% rough ER, 5% smooth ER
- Percentage of total cell membrane: Varies between cells (ER larger in high production cells, mitochondria in very metabolically active cells) Usually surface area goes: rough ER > mitochondrial inner membrane > smooth ER > Mitochondrial outer membrane
- Packing: Very densely packed compartments. Compartments often have reactions happen at the interface where they almost touch. 30% of cytosol is macromolecules
Import into the nucleus (nuclear localization signals):
- Import signal sequence:
- Experiments to discover import or export sequences:
- How does nuclear import occur:
- Import signal sequence:
PPKKKRKV (largely positively charged)
- Experiments to discover import or export sequences:
1: Adding sequence to protein changed where it went.
2: Mutation of the sequence stopped it from being trafficked to original site of localization. - How does nuclear import occur:
1: Nuclear import receptor binds: nuclear import signal and nuclear pore complex proteins (probably nuclear pore cytosolic fibril)
2: Protein with nuclear import signal and nuclear import receptor enter through the NPC (nuclear pore complex)
3: Protein with nuclear import signal dissociates. Nuclear import receptor binds RAN-GTP, which moves it back outside through NPC. RAN-GTP -> RAN-GDP, RAN lets go.
4: Rinse and repeat
Export from the nucleus (nuclear export signals):
- How does nuclear export occur:
- How does nuclear export occur:
1: Nuclear export receptor binds: nuclear import signal and RAN-GTP and nuclear pore complex proteins
2: Exit through the NPC
3: RAN-GTP -> RAN-GDP in cytosol. Protein disassociates, receptor is cycled back into the nucleus
4: Rinse and repeat
RAN:
- Two states and why:
- Two states and why:
Active in GTP state, inactive in GTP state. Phosphorylase present in the cytoplasm (so RAN will be inactive there) Kinase present in nucleus, so RAN is activated there
Import into the mitochondria (mitochondrial localization signals):
- Players:
- Nuclear export:
- How does nuclear import occur:
- Players:
1: Protein with amphiphilic mitochondrial import sequence (Positive on one side): Helps with Entry.
2: TOM complex: recognizes mitochondrial import sequence, feeds protein through into intermitochondrial membrane
3: TIM23: can recognize additional sequence information, and feed it through into the matrix of the cell. sequence is cleaved after it makes it in, so it doesn’t escape out of TIM23.
4: hsp70: which binds to the side entering the mitochondrial matrix, uses ATP and brownian motion as a one way ratchet, and keeps it from diffusing back out.
ER Localization:
- Signal:
- Players:
- Process:
- Signal:
On N-terminus, varies but there is usually a alpha helical hydrophobic core
- Players:
N-terminus, hydrophobic core signal: Recognized by signal recognition particle (SRP)
SRP: has mRNA component. Recognizes ER localization signal, binds to ribosome and GTP, binds to SRP receptor. Eventually cleaves GTP to GDP to disassociate.
SRP receptor: also has GTPase activity in both its alpha and beta subunit. Bound by SRP, goes and finds SEC61 (a translocon (brings through ER membrane)). SRP receptor and SRP allow entry of protein-riposome complex into ER membrane.
SEC61: A translocon, has hydrophobic plug, which is displaced by protein hydrophobic region. Synthesized into membrane through this protein.
Process:
See picture.
1: Note that GTP hydrolysis is necessary for release.
2: Note that Alpha and beta subunit of SRP receptor and SRP GTPase activity.
3: Note ER localization is usually permanent, hard to get back out
4: N-terminus is cleaved to allow protein to float freely into the ER.

How is a signal transmembrane domain inserted into the ER membrane if the N-terminal ER signal is still present?

How is a signal transmembrane domain inserted into the ER membrane with a internal signal sequence?
Note that the charges dictate orientation. A positive charged amino acid on the cytosolyic side, negative in ER.

How is are multiple transmembrane domain inserted into the ER membrane?
Alternating like sewing

Explain biogenesis of tail anchored proteins:
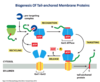
Biogenesis Of Glycosylphosphatidylinositol (Gpi)-anchored Proteins:
Know that this is a valid anchor method. Don’t know factors names. A specific motiff is recognized to signal for this to happen.

Glycosylation Plays An Important Role In Quality Control In The ER explain how:
Misfolded or incompletely folded proteins are glycosylated again, and given the chance to folding again.
Know calnexin’s role in this

Where is the only place bisulfite bridges form?
The lumen of the ER.
If a protein is transcribed by a ribosome floating in the cytoplasm where does it go?
What if the ribosome is attached to the ER?
If a protein is transcribed by a ribosome floating in the cytoplasm where does it go?
1: anywhere, depends on localization signal. (it could even go into the ER)
What if the ribosome is attached to the ER?
It is secreted or part of a plasma membrane, golgi or some vessicle network
What happens to misfolded proteins in the ER?
Transported out of the ER and destroyed.

Three Signaling Pathways That Monitor The ER Luminal Folding Environment:
- Three proteins in pathway:
- What activates them:
- Three proteins in pathway:
Ire1: Expands ER size by lipid and chaperone synthesis
PERK: decreases protein load delivered to the ER lumen
ATF6: Upregulates ER Chaperones (BiP, PDI, Grp94, etc) and cell death
- What activates them:
Chaperones normally bind to them and keep them inactive. The same chaperones bind to and keep misfolded proteins in check. If all chaperones are taking care of misfolded proteins, nothing binds these transmembrane signalling proteins, they leave the membrane and catalyze their reactions.
Cystic fibrosis and quality control:
CFTR has a mutation which makes it fold into a different shape. This shape is destroyed (by transport of ER through a translocase), ubiquination, and proteosome degradation). This misfolded protein still works relatively well but cystic fibrosis results because it is destroyed before it ever meets the cell membrane.


